by Folabomi Oladosu, PhD
Post-doctoral researcher specializing in pain and women’s health at NorthShore University HealthSystem
Every now and again, you wake up to find your arm or leg didn’t quite wake up with you. You dread it, but you wiggle through the pricking, tingling sensations until your once-numb limb is ready to start the day. What if, despite your best efforts, the numbness persists? Numb tingling limbs are one of several hallmark symptoms associated with multiple sclerosis (MS), an autoimmune disorder where the immune system attacks the central nervous system.
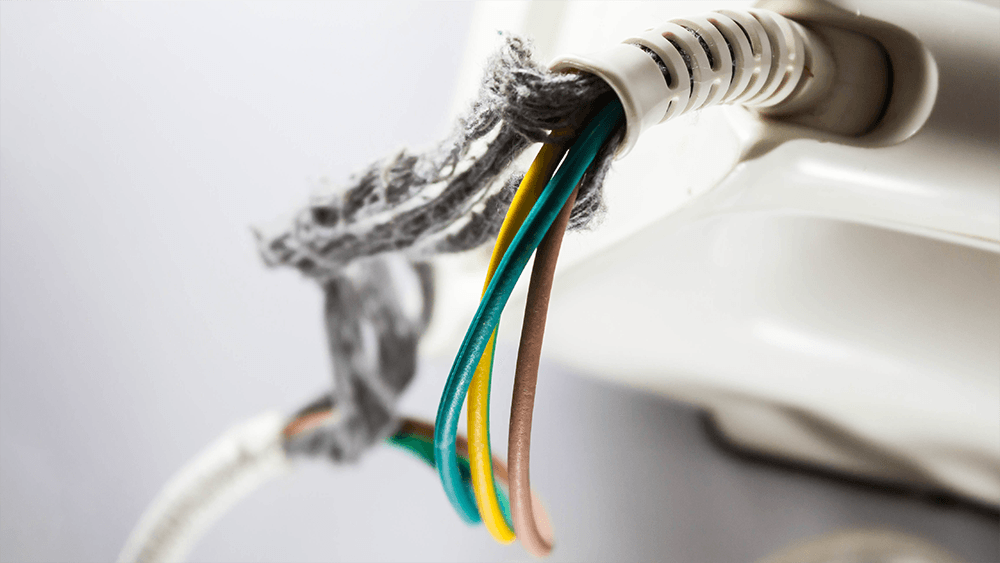
Our nerves are somewhat similar to an electrical power cable. The inside of a nerve is made of smaller nerve fibers. These fibers conduct important electrical signals and messages throughout the body. The outside of the fibers are covered by myelin, a fatty material formed by specialized brain cells. Thanks to its insulating properties, myelin speeds the transmission of electrical messages throughout the body.
If you bend any cable too often, eventually the outer plastic coating starts to fray, exposing the inner metal wiring. The cable may still work, but over time, it becomes less and less reliable (raise your hand if you’re on your third phone power cable). A similar phenomenon occurs in MS. The immune system attacks the body’s own cables, damaging the specialized cells and degrading the insulating myelin on nerve fibers. Over time, this demyelination produces a range of symptoms, including fatigue, depression, pain, and movement issues.
First-line treatments for MS aim to dampen the immune system to reduce demyelination. Although effective, these treatments make the body vulnerable to sickness and infection.
What if there was a way to directly protect the cells and the myelin they produce? Brian Popko and his team wanted to know if Sephin1, a small molecule that enhances a cell’s defensive response to environmental stressors, could protect nerve fibers from demyelination.
Popko uses a mouse model where an injection of myelin proteins coupled with a toxin generates an immune response similar to that in MS patients, initiating a MS-like disease in mice. He and his team then injected the mice with Sephin1 and followed the mice’s responses for 35 days. In this mouse model, clinical symptoms usually appear after 11 days and peak on day 17. When given one week after the initial injection to create MS-like symptoms, Sephin1 delayed disease progression, with clinical symptoms peaking on day 26.
The findings suggest that Sephin1 delayed disease progression by boosting the defensive response to insults from the immune system. When compared to control mice, mice that received Sephin1 had more of the specialized brain cells and less demyelination in the spinal cord on day 17. Sephin1 also dampened immune cell activity in the spinal cord.
The team went on to discover that Sephin1 worked even better when it was paired with interferon beta (IFN-β), an anti-viral protein native to the body, also used as a common treatment for MS. Mice that received both Sephin1 and IFN-β showed much slower, less severe disease progression.
This timely translational research from the Popko lab illustrates the promising therapeutic effects of Sephin1 for MS, especially when combined with IFN-β. Its demonstrated efficacy in MS mouse model indicates Sephin1 may help to protect indispensable nerve fibers from demyelination in MS patients. Dr Popko is currently exploring opportunities to assess the therapeutic safety of Sephin1 for MS patients.