
Jun 29, 2018 | Big Data, Microbiome, News Roundup, Research
A selection of health news from the University of Chicago and around the globe curated just for you.
Research at Shedd Aquarium explores how environment impacts microbiome of dolphins
Research conducted at the Shedd Aquarium with UChicago revealed new details about the microbiome of Pacific white-sided dolphins at the aquarium and how it is influenced by the surrounding environment. (The Forefront)
Can a daily pill prevent kidney stones?
Hatim Hassan and other researchers at Oxalo Therapeutics are developing a drug that uses the microbiome to prevent kidney stones. (Crain’s Chicago Business)
Viruses love what we’ve done with the planet
The more of the planet humans take over, the more we inadvertently make it a viral paradise, and a dangerous place for us to live. (Quartz)
Probiotic, supplement combo extends fruit fly lifespan
A combination of probiotics and an herbal supplement called Triphala extended the lives of fruit flies by 60 percent, report researchers. (Futurity)
Students and UChicago scientists turn Wrigley Field into data lab
Lane Tech students came to Wrigley Field to help UChicago-Argonne researchers install sensors for Array of Things, a project that collects environmental data. (UChicago News)
Apr 23, 2018 | Big Data
by Elise Wachspress
Every year, over a quarter of a million Americans die from sepsis, more than the number of those felled by prostate cancer, breast cancer, and AIDS combined.
Sepsis occurs when the body mounts an overwhelming—and disordered—response to an infection. This cascade of rapidly escalating immune warfare can interrupt blood flow, create systemic oxygen deficits, precipitate blood clots, and damage and shut down multiple organ systems, all at once.
The scariest part of diagnosing and treating sepsis is how fast the condition can overwhelm even the healthiest of patients, particularly since there are no drugs other than antibiotics to treat the disease. While many intensive care physicians are now aggressive about launching immediate and sustained treatment “bundles” against the condition, it is hard to recognize the moment-to-moment responses being mounted by the patient’s own immune system. And synchronizing your battle plans with your ally, or even knowing how you can help, can mean the difference between life and death.
Gary An, MD, co-director of the Surgical Intensive Care Unit at the University of Chicago Medicine, and fellow faculty member Chase Cockrell, PhD, decided to look for ways to get out ahead of this runaway train. They constructed a simplified computational model of the human immune system, simulating how immune cells and signaling molecules behave during sepsis, which allowed them to observe how the model responded to various potential interventions applied in the first week of a clinical course.
The model they built clearly could be useful for future patients, particularly in terms of driving the development of new drugs and patient sensors. What if one could seed the computer model with data from a patient newly diagnosed with sepsis and then game out the best treatment strategies in silico? Computer modeling might rapidly provide an individualized care strategy honed to the needs of that patient, with less trial and error. By identifying the right “prescriptions” early, we could save many more lives.
Right now, individualized modeling is not cheap; An and Cockrell’s approach required up to $100,000 in computing time. But as of 2013, sepsis was the most expensive condition treated in US hospitals, with an aggregate cost of $23.6 billion for nearly 1.3 million hospitalizations. In fact, the costs of hospital stays for sepsis have more than quadrupled in just the past two decades. And by creating a machine-learning algorithm, perhaps the costs of what An and Cockrell describe as “adaptive personalized medicine” could be reduced significantly. An believes this computational approach might help address some of medicine’s biggest problems.
Can we predict patients’ susceptibility to sepsis?
Another UChicago team used computational approaches to understand the factors that put patients at risk of sepsis in the first place, and in doing so identified some strategies that might help patients better survive the disease.
A team led by Philip Verhoef, MD, PhD, explored insurance records from over 70,000 patients across the US treated for sepsis to understand how the structure of patients’ immune systems affected whether they were likely to succumb to the disease. The team found that patients with certain auto-immune diseases—ulcerative colitis, multiple sclerosis, type 1 diabetes, or lupus—were at greater vulnerability for sepsis, a finding that was not totally surprising. What was more unexpected was that patients whose immune conditions were linked to Th2 cells—a specific type of immune cells that are overactive in diseases like asthma, dermatitis, or allergies—were much less likely to have been treated for sepsis.
The team then set out to understand why this was true. They subjected mice to an infection with a bacterium known to cause a sepsis-like inflammatory cascade. But a few days before they did so, the team injected half the mice with an agent known to invoke the Th2-cell (allergic) response. While over 75 percent of the mice who received the injection survived, a full 85 percent of those who did not died.
This later experiment seems to have surfaced a completely novel disease mechanism—that Th2 cells might be especially useful in fighting sepsis—one that scientists and physicians might exploit to improve sepsis outcomes.
Mobilizing computational tools, as scientists did in these two research projects, stands to open up an entirely new approach to biomedical discovery. The robust investments in data handling and analysis being made through the Duchossois Family Institute stand to make a significant impact in helping people live longer, healthier lives.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office
Mar 9, 2018 | Big Data, Vaccination
by Elise Wachspress
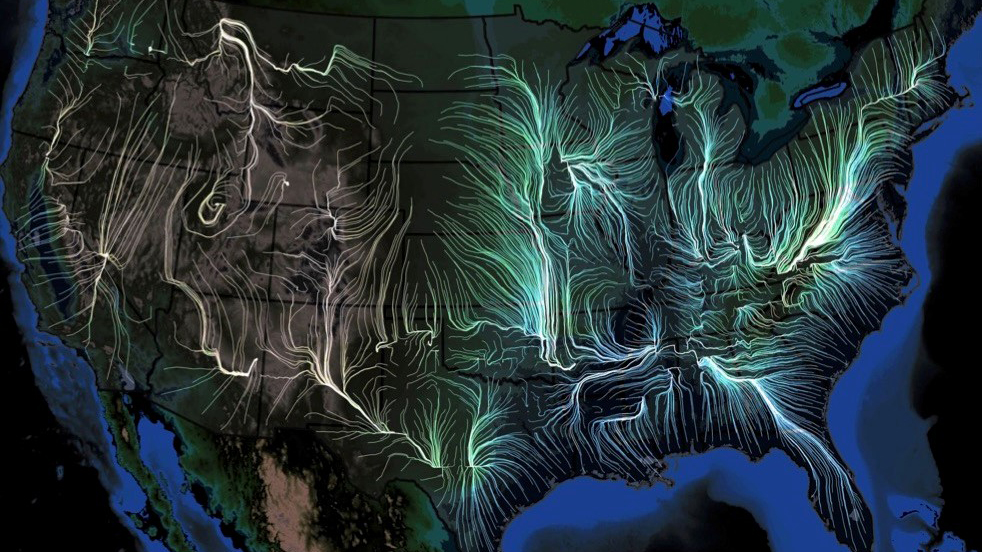
Last week, the scientific journal eLife (established by the Howard Hughes Medical Institute, Max Planck Society, and the Wellcome Trust) published a remarkable study—a veritable “weather forecasting” tool to predict, in detail, the movement of the flu in the U.S.
Scientists from the University of Chicago, Microsoft Research, Columbia University, and Argonne National Laboratory used many gigantic, disparate datasets—insurance records of more than 150 million people, 1.7 billion geo-located Twitter posts, air traffic, weather, how often people visit friends nearby, vaccination rates, even mutations in the virus itself—over nine flu seasons, from 2003 to 2011, to create a model to describe in detail how flu epidemics start and spread.
The team then verified the accuracy of their computational model by “predicting” the spread of flu for three subsequent seasons (2014-2017), predictions which dovetailed with what actually happened, at resolutions higher than those created by the Centers for Disease Control and Prevention.
The scientists’ work documented that seasonal flu outbreaks generally originate in warm, humid areas of the south and southeast, mostly near the Gulf of Mexico or the Atlantic Ocean; though infections can originate in many places, they usually gain traction and grow into epidemics only near large bodies of water. Of the factors most important in spreading the virus beyond these coastal areas, foremost was the original host population’s “social connectedness”—how often people interacted on a day-to-day basis. The next most critical factors were weather (high humidity, high temperature, and low barometric pressure fostered the epidemic); changes in the virus itself; and land travel by the host population, which turned out to be much more powerful in fueling an epidemic than airplane travel.
The new model they derived offers a great tool to help public health officials improve prevention efforts, suggesting, perhaps, how best to deploy flu shots early on, and how people near the coasts should think about their local travel and socialization once people start getting sick. In fact, the model could be used to test prospective public health policies in silico before they are implemented, saving precious energy and resources.
But perhaps as interesting as the forecasting tool is the scientific “network” that created it.
The strength of weak ties
When you are looking for a job, identifying potential business investors, even finding a play group for your kids, it’s unlikely your spouse, siblings, or even immediate friends can help much. They know many of the same people and ideas you do. We’ve learned the power of networking, reaching out to those somewhat distant from us, for better answers. The internet and social media like Pinterest and YouTube have brought networking to a whole new level: they provide easy access to all kinds of far-flung, elusive information, from how to help your kids with their math homework or make crepe suzettes, to how to do your taxes or evaluate your investment portfolio.
Networks work most effectively when they include lots of weak, distant ties. The more people from disparate backgrounds you can reach even briefly or intermittently, the more likely you are to gain more knowledge and better ideas.
Andrey Rzhetsky, PhD, the leader of the flu model team, has mastered the art and science of networking.
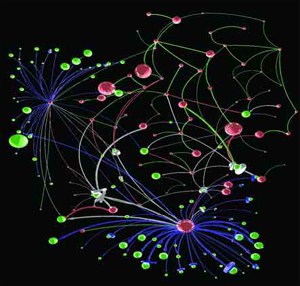
Rzhetsky’s network map of patterns of gene expression in brain tissue of people with autism, from a 2010 paper
He began his career as a mathematical biologist, analyzing gene sequences and connecting the dots, when computational biology was still in its infancy. By 1991 he was harnessing microcomputer programs to construct “phylogenetic trees”—categorizing differences among organisms—to better understand evolution. As more and more scientific research was published online, Rzhetsky became among the earliest developers of text mining, an automated way to search journal articles, capturing and cataloguing connections between genes, proteins, and diseases, without a human having to read each article.
He began training machines to suss out connections between all kinds of data. The “networks” of information he built ranged from molecular interactions in biological systems, to documenting how seemingly unrelated diseases overlap, to the reliability of scientific studies and much more, informing a growing variety of fields. What environmental factors might trigger autism or bipolar disorder? Which diseases inflict the greatest human suffering? How should scientists choose experiments to best accelerate the progress of science and medicine? Rzhetsky and his collaborators have mined and harnessed data to find useful answers to these disparate questions and more.
And the number and diversity of his collaborators on these projects is pretty stunning. Rzhetsky is ingenious at using weak ties to find expertise all over the world. Whether it is getting access to huge caches of medical records that stretch over generations (Denmark) or computational/artificial intelligence experts at major corporations (Seattle), Rzhetsky uses the power of weak ties to build bases of knowledge that push the boundaries of science. His cohort on the flu research provides an example:
- Rzhetsky’s closest partner was Ishanu Chattopadhyay, PhD, now also a faculty member at UChicago. Chattopadhyay has developed algorithms for a broad swath of areas: path-planning for mobile robots, autonomous decision-making, swarming behavior and self-organization, and reverse-engineering measurements of populations, species, and gene expression to understand systems’ mechanisms.
- Josh Elliott, PhD, started his career as a high-energy theoretical particle physicist. Over the past decade he’s applied those analytical skills to climate change, agricultural production, economic modeling, and the social sciences as a research scientist in the University of Chicago’s Energy Policy Institute.
- Emre Kiciman, PhD, a principal researcher in artificial intelligence at Microsoft Research, lent his expertise in social media analysis, particularly attuned to its inherent systematic biases.
- And Jeffrey Shaman, PhD, of Columbia University, provided proficiency in climate, atmospheric science, hydrology, and other environmental determinants of infectious disease transmission and forecast.
Scientific successes like theirs demonstrate that novel breakthroughs depend more and more on teams with diverse expertise, experiences, and viewpoints. Just as casting a wide net leads us to new recipes (and ways to avoid the flu!), so those on the forefront of discovery must reach across departments, institutions, even national boundaries to achieve the greatest knowledge.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office







