Celiac disease: It’s all in the mix
by Elise Wachspress
There was a time when many people thought that unlocking the genetic code would help us easily identify how diseases arose and better strategies for treating or preventing them.
And that was true for the very few diseases precipitated by individual genes, like cystic fibrosis. Single-gene diseases, however, are fairly uncommon, because over time, especially when they interfere with reproduction, natural selection has been pretty effective in weeding them out of the “gene pool.”
Most diseases are “complex,” involving the contribution and interactions of many genes. An explosion of genetic studies over the past couple of decades suggests most genes contribute only a small degree of disease risk. Thanks to the redundancy built into the human body through eons of evolution, those who carried one or even several “disease genes” would likely never develop the disease.
And even those at very high genetic risk were often disease-free. Researchers began to suspect that some kind of environmental trigger was necessary to activate some disease mechanisms—putting us right back at a (much more complicated) version of the “nature vs. nurture” dilemma.
Environmental triggers can be hard to recognize or assess. Methods for evaluating air and water quality, better food labeling, even sophisticated wearable trackers are helping us identify some potential environmental factors, but there are many others we might not even have considered.
Disease caused by microbes: The other side of the coin
Long before genetic testing, we knew that exposure to certain viruses and bacteria also caused diseases, often independent of our genetic makeup. Polio, measles, rubella, and others were shown to be caused by a single type of microbe, like some diseases were caused by single genes. Again, this simplified the strategy for solving these: scientists developed vaccines, a major medical success story.
Now it is clear that some diseases arise from combinations of bacteria—or combinations of genes and bacteria. Like any puzzle, the more “unknowns” involved, the more complex the problem becomes.
Celiac disease is one very complex problem.
A (painful) gut reaction
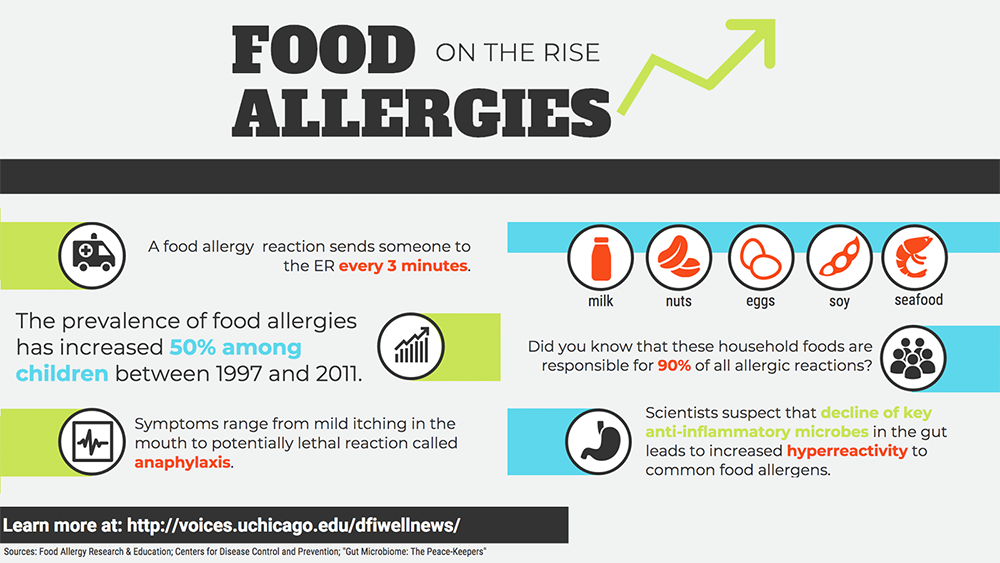
Estimated to affect one in 100 people worldwide—two-and-a-half million in the U.S. alone—celiac is a serious autoimmune disorder that damages the small intestine, causing diarrhea, fatigue, weight loss, anemia, and sometimes an itchy, blistering rash. With celiac, the gut can no longer effectively absorb nutrients; in children, the condition can significantly retard growth.
Initially, celiac causes this damage only in the presence of gluten, found in wheat, rye, and barley. Unfortunately, since these grains have sustained humans for millennia, gluten is ubiquitous, not just in food, but also vitamins, hair and skin products, even toothpaste. For those with celiac, avoiding gluten imposes a heavy burden, and reading labels becomes a family sport. Indeed, it is common to find whole families suffering from the condition, as those with a parent, child, or sibling with celiac have a risk as high as one in ten of developing the disease. And for 40 percent of adults whose systems are already damaged, even avoiding gluten allows for only a partial recovery.
Scientists have pinpointed two genes associated with celiac, but even if you have both, your likelihood of developing the disease is only 3 percent. Bana Jabri, MD, PhD, and her team at the University of Chicago were convinced there must be some other trigger involved. Because celiac is an autoimmune disease, they thought a microbe might be a likely candidate.
In studies of both mice and humans with “celiac genes,” they found that a reovirus infection, which causes no other symptoms, could break the body’s ability to tolerate gluten and initiate the pathological celiac response. Thus, it likely takes genes coupled with exposure to a particular virus to trigger the autoimmunity—one reason why incidence even within families is lower than might be expected.
Preventing celiac—and perhaps other diseases
This information gives us new potential strategies for gaining control over the disease. Since children lose their maternal antibodies against reovirus around six to nine months of age, introducing gluten to a baby’s diet outside this window might reduce the chances of getting celiac. And vaccinating children at genetic risk against the virus before they first eat gluten might also keep them disease free.
On a scientific level, this study has broader ramifications. It demonstrates that a clinically silent virus—not a usual suspect—can cause a lifelong, pathogenic inflammatory response to an otherwise harmless substance. So environmental factors that seem innocuous can, in combination with genes or other factors, cause some unexpected and serious outcomes. Like a recipe or a team, it’s all in the mix.
As in so many cases, basic science research like Jabri’s provides broad and surprising insights into not just one particular disease or drug, but how our bodies work as a system. It is these kinds of discoveries that can change our whole approach to health and disease.
There is a simple blood screening available for celiac disease. You can schedule an appointment with the University of Chicago Celiac Disease Center at 1-888-824-0200.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office