Nov 22, 2017 | Heart Disease, Microbiome
by Elise Wachspress
Peripheral artery disease (PAD), the narrowing of the blood vessels in the body outside the heart and brain, affects more than 2 million adults in the United States.
PAD occurs when plaque—an amalgam of fat, cholesterol, calcium, and other substances—builds up on the walls of the arteries to the arms and legs. These corroded “pipes” can restrict or even stop blood flow, particularly in the legs and feet.
The person with PAD may feel no symptoms, but many begin to notice that walking or climbing stairs causes leg pain or numbness which stops on resting. The skin on their feet and legs may look pale, blue, or shiny, and nail and hair growth often slows or stops completely—all indications that oxygen and nourishment are not getting where they need to go.
Over time, people with PAD have increasing difficulty with walking; they are more likely to lose their independence, with major impact on quality of life. Those with very advanced disease may develop sores on the feet and legs, which often heal slowly, or not at all. They are also at risk for problems in the arteries in the heart (coronary artery disease) and in the brain (cerebrovascular disease). Because of the strong connection between plaque in the legs and other parts of the body, PAD is one of the largest risk factors for both heart attack and stroke.
While the disease can start as early as age 40, incidence increases significantly with age. By age 70, more than ten percent of men and nearly as many women are dealing with PAD. For African Americans, the numbers are nearly twice as high.
Clinicians know that smoking, high blood pressure, high cholesterol, and diabetes can set up PAD, but not everyone with these risk factors develop the disease. Physician-scientists are beginning to understand that inflammation—the body’s complex immune response developed to protect against microbial pathogens or other irritants—is also a major player, causing plaque to develop inside the artery walls. But how to pinpoint the irritants that invoke this response? There are millions of kinds of microbes in, on, and around us that could be causing this immune response, but the vast majority—especially those in our gut—are essential for keeping us healthy.
University of Chicago scientists engaged in the fight against PAD are now focusing on the complex interplay of the immune system, inflammation, and the microbiome. Better understanding of the mechanisms involved may yield new tools and strategies against the disease and the heart attacks and strokes that can follow.
The researchers are coming at this problem from three angles: in the lab, in the clinic, and in the broader population.
- In the lab, they are feeding mice high-cholesterol diets to see how the microbial balance in each mouse affects the development of plaque in the arteries. By then transplanting fecal matter from healthy humans into mice with PAD, the team can see if changing the bacterial balance in these mice slows or stops disease progression.
- In the clinic, the team is tracking patients with serious PAD, those with wounds so severe they must have surgery to improve blood flow in hopes of preventing amputations. The team will track how the balance of bacteria, both in the patients’ gut and on their skin, affects blood flow to damaged tissues. By pairing extensive clinical data about these patients—including measures of inflammation in the blood and studies of genetic information —with information on the microbial populations present in each patient, the team aims to identify the balance of bacteria that promotes healing.
- In the wider population, the investigators aim to track 100 patients with PAD over two years. In this much broader group, they hope to discover the connections among each patient’s microbiome, immunological markers, imaging tests that measure arterial health, and, importantly, functional status—through a walking test—to gauge how the microbiome affects not only disease progress but also quality of life. The investigators will also track the participants’ diet and physical activity, two factors well known to influence the microbiome and PAD. Because UChicago Medicine has developed relationships with patients of such diverse ethnic, racial, and economic backgrounds, this study can yield findings to suggest new treatment strategies for a wide spectrum of individuals.
Each of these three studies will inform the others. This three-pronged approach is a classic example of how academic research drives better medicine: 1) find ways to get at the underlying biological mechanisms of health and disease; 2) translate that understanding to address the critical problems of the sickest patients, and 3) develop the knowledge that can help the broader population achieve wellness and the most satisfying life possible.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office
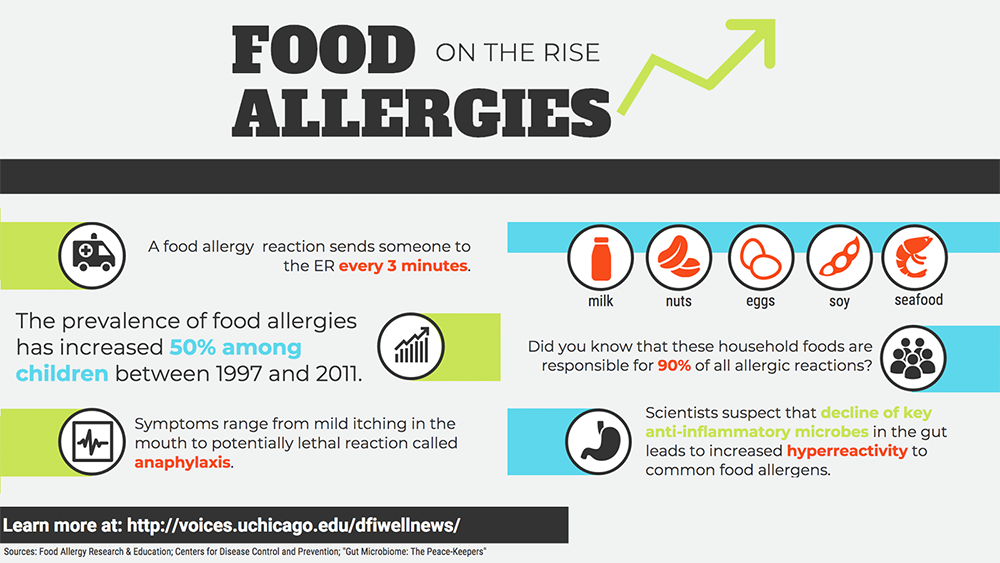
Nov 15, 2017 | Food Allergies, Microbiome
by Maggie Zhang
Graduate student in the Committee on Microbiology
Food allergies constitute a major public health concern. For an estimated 15 million Americans, exposure to common foods such as peanuts and milk causes negative, even deadly, immune responses. In recent years, food allergy rates among children have risen sharply, increasing approximately 50 percent between 1997 and 2011.
Why is the problem growing?
Let’s go back to the nineteenth century, when the findings of Louis Pasteur, Joseph Lister, and many others were converging on an essential function of the human body: immunity. The immune system was so named because it seemed to “exempt” the body from attack by microorganisms.
Given that microbial infection was thought to be the primary cause of immune reactions, it seems counterintuitive that people today—more free from infectious disease than ever—would be so heavily crippled by inflammation, the quintessential immune response. But decades of research reveal that a hyper-reactive immune system underlies autoimmune and allergic diseases, leading to severe conditions such as anaphylaxis.
Anaphylaxis can be life-threatening. In the same way that a healthy immune system reacts robustly to the intrusion of foreign toxins and microbes, a hyper-reactive one can respond just as dramatically to certain foods.
Despite the increasing prevalence of food allergies, current treatment options are problematic. Searching for new solutions, scientists have turned to the gut microbiome, the teeming ecosystem of microscopic organisms occupying our gastrointestinal system.
For some 800 million years, we have been building mutually beneficial relationships with the microbes that dwell within our gut. But over the past few decades we have drastically altered the environment that they call home, thanks to widespread antibiotic administration, extreme sanitary practices, and especially a high-fat, low-fiber diet.
Our dietary choices affect the species of bacteria that live within us. Our microbes eat what we eat, taking a small cut of our food in exchange for synthesizing nutrients that we need but cannot make ourselves. Many bacteria ferment what we cannot digest, such as the soluble fibers in vegetables, fruits, grains, and legumes. As a byproduct, they produce anti-inflammatory molecules called short-chain fatty acids.
Bacteria known as Clostridia are star performers. Cathryn Nagler, PhD, and her team have pinpointed Clostridia as key peacekeepers in the gut microbiome. Using mice born and raised in a microbe-free environment, Nagler’s group has demonstrated that introducing Clostridia blocks sensitization to food allergens. The microbes foster an anti-inflammatory environment within the gut in multiple ways, promoting the secretion of mucus along the gastrointestinal lining and producing a short-chain fatty acid called butyrate, which nourishes cells in the colon. These help to create a protective barrier in the gut, which prevents allergens—like peanuts and milk—from encountering pro-inflammatory immune cells.
These findings might soon change how we treat allergies. Nagler has teamed up with Jeffrey Hubbell, PhD, of the University of Chicago’s Institute for Molecular Engineering, to launch the start-up ClostraBio in order to commercialize novel food allergy remedies. Their aim is to develop novel, targeted treatments to restore the protective barrier naturally provided by peacekeeper microbes.
For centuries, we have appreciated the protective functions of the immune system against microbial attack. We have memorialized the contributions of Pasteur and Lister in everyday words like pasteurization and Listerine®, concentrating on the harmful bacteria that we must eliminate to remain healthy.
But in fact, only about 2 percent of all microbes are pathogenic—the rest are neutral, beneficial, or even essential to our well-being. While there is no doubt that modern sanitary practices have reduced the scourge of infectious disease, we have come to understand that though “bad” microbes can cause disease, “good” bacteria can also prevent it.
Just as plants depend on microbes to extract vital nutrients from the soil, scientists now hypothesize that animals only achieved mobility when we learned to carry within our bodies the microbes necessary for our survival. Maybe it’s high time for us to appreciate the importance of our symbiotic pact with the microbes that helped make us who we are today.

Nov 9, 2017 | Microbiome
by Renée de Pooter, PhD
Staff scientist in the Department of Pathology
What do Natalie Cole, Dick Cheney, and Jim Nabors have in common?
Like a growing number of Americans—33,600 in 2016—they received life-saving organ transplants. When vital organs like the lungs, heart, liver, or kidneys are destroyed by disease or genetic conditions, organ transplantation is the only cure. Many of these organs come from people who sign their organ donor cards, in the hopes that even in tragedy they might be able to save someone else’s life. Marisa Alegre, MD, PhD, and her team at the University of Chicago are trying to learn how to prolong survival for both the transplanted organ and the recipient, to get the most benefit from this final, amazing gift of life.
Surgical recovery is only the beginning for transplant recipients, because the life-saving organ comes from another person and their bodies know it. Our immune systems evolved to protect us from infections, but some microbes can burrow inside our cells to avoid detection. To leave clues about where these stealthy invaders are hiding, infected cells leave “breadcrumbs” on their surfaces, by-products of what the invaders are making inside. Like drug-sniffing dogs on a raid, immune cells hunt down these clues in the body’s nooks and crannies. If they find something unfamiliar, they howl and send the DEA agents, the T cells, into defensive action.
It’s a great system, except when someone receives an organ transplant.
Then the immune system attacks the foreign cells, thinking it’s fighting a massive infection. To pacify the immune system, organ recipients face a lifetime of immunosuppressant drugs. Unfortunately, this stupefies their T cells and leaves the recipient vulnerable to infection and cancer.
In rare cases, the immune system and the transplanted tissue reach a truce. In these “tolerant” individuals, the new organ survives without the immunosuppressive drugs and the immune system still protects against disease. Clinicians usually find these outliers by accident: the patient stops taking his or her immunosuppressants for medical or financial reasons and yet still survives, perhaps even thrives.
Many researchers, including Alegre, took note of these lucky unicorns. They reasoned that if we better understood how these patients became and stayed tolerant, we might be able to improve outcomes for many more transplant recipients.
Several years ago, the teams of Alegre and Anita Chong, PhD, showed that severe bacterial infections could cause transplant rejection in previously tolerant mice. Then, they set out to test how the microbiome – the collection of bacteria that live on the mice – affects transplant survival.
First, they treated mice with antibiotics. This didn’t wipe out the entire microbiome, but killed off all the antibiotic-sensitive members. They found that treating both donor and recipient nearly doubled the survival time of the transplant.
Next they looked at germ-free mice, special experimental animals born and raised in bacteria-free bubbles—an exceptionally expensive but irreplaceably useful laboratory technique. As with antibiotic-treated mice, foreign tissue transplanted in germ-free mice lasted longer than usual.
The team then exploited an icky but valuable characteristic of mice: they eat poop. Alegre’s team fed the germ-free mice the poop of mice with a normal microbiome: a fecal transplant. Germ-free mice that were fed normal mouse poop rejected the organ grafts as quickly as the normal mice. But when the germ-free mice got poop from antibiotic-treated normal mice, they rejected the organ graft as slowly as their brethren who remained bacteria-free. It appears that at least one of the normal components of the mouse’s microbiome—one sensitive to the antibiotics—was causing the immune system to reject the new organ faster.
This work suggests that we might be able to increase transplant tolerance in humans by treating them with specific antibiotics. And if we can discover the exact bacteria causing the rejection, we might be able to use a short-term antibiotic targeted very directly at eradicating the trouble-makers, perhaps even in the case of organs harvested after a donor’s death.
Weeding out the microbial agitators while leaving most of the patients’ microbiome intact may allow doctors to limit the immunosuppressants prescribed and help keep their patients healthy for a new life with a new organ.

Nov 2, 2017 | Microbiome
by Maggie Zhang
Graduate student in the Committee on Microbiology
In 1683, a Dutch merchant named Leeuwenhoek built his own microscope and focused it on some residue he found on his teeth. What he saw prompted him to fire off a letter to the Royal Society of London, describing the “animalcules” he realized were living in his mouth.
We now appreciate that these microbial creatures are everywhere on Earth, thriving even in hostile environments the likes of which other animals could never tolerate—underneath Antarctic glaciers, within bubbling asphalt lakes, inside hydrogen sulfide gas caves. Some, like Leeuwenhoek’s “animalcules,” grow just fine in the presence of oxygen; other “anaerobes” can’t tolerate air at all.
Bacteria are important members of every ecological niche. We like to give plants all the glory for producing the oxygen necessary for life, but photosynthetic marine and freshwater inhabitants actually produce half of the oxygen we breathe. Meanwhile, bacteria living in the soil are often model citizens, breaking down nature’s waste to obtain nutrients for themselves and recycling the rest into building blocks for new life. What’s more, the human gastrointestinal tract houses over three pounds of bacteria that break down our food and provide us with essential vitamins.
With the fundamental roles that microbes play in our daily lives, it’s important for us to understand them. But how do you physically and genetically dissect something so small?
Scientists initially solved this problem by growing individual strains of bacteria in rich broths to study their biological properties, including their DNA. Soon enough, they ran into a roadblock: less than 1 percent of the bacteria on Earth could be easily cultivated. Studying the other 99 percent was going to require some new tools.
Thus, the field of metagenomics was born. Metagenomics technologies allow us to directly sequence the entire genetic contents of all microbes living within a habitat.
Unfortunately, sequencing the collective genetic makeup of even a 2,000-member community means several million DNA fragments. Although accurately piecing them back together into coherent genomes provides essential insights to microbiologists, the task is not much different than simultaneously assembling 2,000 jigsaw puzzles with all the pieces mixed into one huge pile.
Yikes.
A. Murat Eren, PhD—known to colleagues as Meren—and his team at the University of Chicago are at the frontier of developing the computational tools necessary for this daunting task. They have created several flexible, open-source software packages that allow users to dig deep into their data while retaining command of their analysis. These tools enable scientists to reconstruct novel microbial genomes from environmental samples, study their evolutionary relationships to other organisms, and investigate their abundance and distribution, as well as their functions. Collectively, the approach can tell us a lot about the biological traits important for a given microbe to survive and promote various environmental conditions, including health and disease.
In 2015 Meren and his team launched anvi’o, an advanced analysis and visualization platform. Using this software, scientists can ask a wide range of questions about the metagenomes they are studying through a highly intuitive, fully customizable interactive interface. The team has produced many highly comprehensive tutorials for users.
Anvi’o has already been cited by more than 50 publications, ranging from studies characterizing the human microbiome to articles debating the origins of humankind. Meren and his team make the software freely available online, for researchers all over the world.
At UChicago and affiliated labs at the Marine Biological Laboratory in Woods Hole, Massachusetts, and at Argonne National Laboratory, Meren’s tools are helping colleagues re-envision the future of microbiome research. Scientists are using the tools to study the use of antibiotics during pregnancy, fight serious infections, understand gut inflammation, and even survey oral “animalcules” much like those Leeuwenhoek saw in his mouth centuries ago.
This research would be impossible without scientists like Meren who also excel in computational technologies. With outstanding tools at the centerpiece of the efforts, these collaborative efforts will help us gain knowledge about the microbiome, which will improve health for millions.
As it has been through eons of human history, scientific discovery and tool development are like the chicken and the egg, totally dependent on each other. But Meren and his team put the scientific chicken first: “Although the inherent link between the tool and thinking will continue to bind them together, we believe it must be mostly the intellectual curiosity that drives the direction of science, and not the comfort of what is available. We intend to maintain our flexibility, and let the incoming questions shape and re-shape our software.”

Oct 9, 2017 | Diabetes, Microbiome
by Elise Wachspress
In September 2008, Nature published a feature story hailing the work of Alexander Chervonsky, PhD, and his team at the University of Chicago. They had recently found startling evidence that the microbiome—the complex community of trillions of microbes living in our guts, skin, and elsewhere—could provide an explanation for rising rates of type 1 diabetes, particularly in developed countries.
While there is clearly a genetic component to the disease, the team knew there must be other factors at work, since genetic change in human populations just doesn’t happen that quickly.
Chervonsky’s team hypothesized that a protein named MyD88, which regulates immune responses to resident microbes, was a player. So they genetically engineered mice prone to type 1 diabetes but without the gene that encoded MyD88.
None of these “knock-out” mice developed the disease. But, amazingly, when the knock-out mice were raised in an environment without bacteria, they did.
And when Chervonsky’s team looked more closely at mice with the MyD88 gene, they realized the microbiome of those mice was actually different than the knock-out mice. The MyD88 gene was actually tilting the balance of the gut microbial composition to promote diabetes.
Clearly, the combination of gut microbes and the gene MyD88 was somehow involved in type 1 diabetes. And these effects were being felt in the pancreas, not in the gut, where the microbes lived. What were the mechanisms that caused these outcomes? Follow-up studies in Chervonsky lab suggested that a combination of the mice’s genetic makeup and the composition of the microbiota in their environment could shift the balance from health to disease.
Opening a whole new area of science
The “ecosystems” we inhabit include not only plants and animals, air and water. We are profoundly affected by the trillions of microbes living in, on, and around us as well. We may not be able to see them, but our genomes have been co-evolving with these tiny organisms for millennia—and they may be powerful agents in maximizing our health and well-being, protecting us against disease as well as occasionally causing it.
Thanks in part to basic research by Chervonsky and his team, there has been an explosion of studies linking the microbiome and type 1 diabetes. JDRF, the leading global organization focused on type 1 diabetes research, has reoriented significant funding toward microbial approaches. Recently, large longitudinal studies on babies—humans, not mice—show that a drop in microbial diversity seems consistently to precede development of the disease.
By looking closely at the stool samples of babies like these, we may discover the bacterial mix that can keep others from developing disease. Perhaps introducing these bacteria to babies at risk could assure they remain free of type 1 diabetes—an onerous, life-long condition that requires constant monitoring, and infusion or injections of insulin strictly balanced with eating and exercise. Or what if we isolate the protective bacterial by-products and add to infant formula or yogurt?
Getting safely to effective clinical applications is not a simple process; the ethical considerations alone are far more complex than any work with baby mice. But the current costs exacted by type 1 diabetes—in medical care, lost school and work time, and most importantly, quality of life—make investing in this avenue of discovery as valuable as, say, new surgical techniques.
The Duchossois Family Institute: Harnessing the Microbiome and Immunity for Human Health, is founded on that premise: that understanding how these two forces—the microbiome and immunity— work together can yield a new foundation for robust, life-long wellness. Baked into the Institute’s plan are advanced data collection and computational analysis and the business strategies that can accelerate breakthroughs like those made by Chervonsky’s team. The DFI will start from basic science research like his—absolutely essential to understanding biological systems—and move findings rapidly to clinical application and the commercialization that makes these accessible to many around the world.
We hope you will be a frequent visitor to this blog as we demonstrate growing understanding of the role of the microbiome in allergy, Alzheimer’s, hypertension, obesity, cancer, celiac disease, sleep, lung conditions, neurodevelopmental conditions, and more.
Join us for what we believe will be an amazing journey.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office