Novel Vaccines and Vaccination Strategies
Vaccines delivered through the mucosal route are known to induce T helper type 17 (Th17) responses and provide superior protection against Mtb infection. However, already tested Th17-inducing mucosal adjuvants such as heat labile enterotoxins and cholera toxins, are not considered safe for use in humans. To address these issues we have rationally screened adjuvants and identified combined use of MPL and chitosan as a novel formulation for use as a mucosal adjuvant to generate protective immune responses against Mtb infection. The fact that this vaccine formulation is protective against emerging clinically relevant Mtb strains such as the hypervirulent Mtb HN878 strain, further supports the use of MPL-chitosan as a potent Th17 inducing adjuvant for mucosal use in human TB vaccines.
As the development of safe mucosal adjuvants for human use is critical, we have further demonstrated that nanoemulsion (NE)-based adjuvants when delivered intranasally along with Mtb specific immunodominant antigens (NE-TB vaccine) induce potent mucosal IL-17 T-cell responses. Additionally, the NE-TB vaccine confers significant protection against Mtb infection, and when delivered along with BCG, is associated with decreased disease severity (Ahmed et al. 2017). These findings strongly support the development of a NE-TB vaccine as a novel, safe and effective, first-of-kind IL-17 inducing mucosal vaccine for potential use in humans. The NE-TB technology has been submitted for a patent at the Office of Technology Management – Washington University in St. Louis. Further investigations and trials with primate and guinea-pig models are ongoing and will be very crucial toward achieving higher goals and translation of the NE-TB vaccine for use in a human TB vaccine.
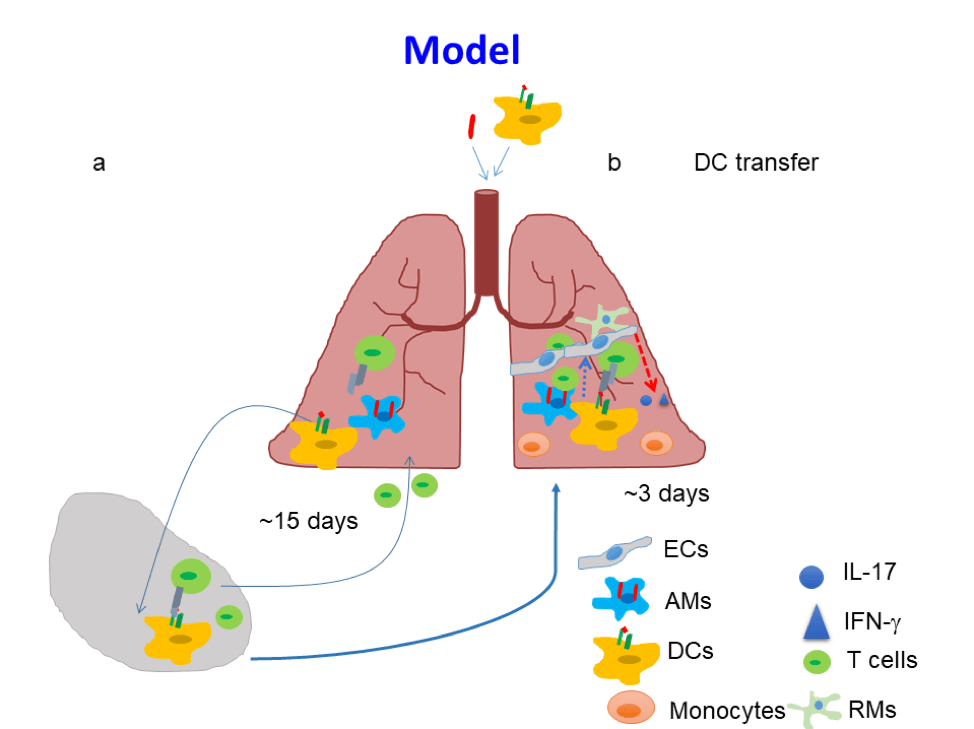
The development of a TB vaccine that induces sterilizing immunity to Mtb infection has been elusive. Absence of sterilizing immunity induced by TB vaccines may be due to delayed activation of mucosal DCs, and subsequent delay in antigen presentation and activation of vaccine-induced CD4+T-cell responses. Our published studies have shown that pulmonary delivery of activated Mtb antigen-primed DCs into vaccinated mice, at the time of Mtb exposure, can overcome the delay in accumulation of vaccine-induced CD4+ T-cell responses. In addition, activating endogenous host CD103+ DCs and the CD40-CD40L pathway can similarly induce rapid accumulation of vaccine-induced lung CD4+T-cell responses and limit early Mtb growth (Griffiths et al. 2016). Thus, targeting mucosal DCs can accelerate vaccine induced T-cell responses on Mtb infection, and provide insights to overcome bottlenecks in TB vaccine efficacy.