The Non-myelinating Phenotype of Oligodendrocytes
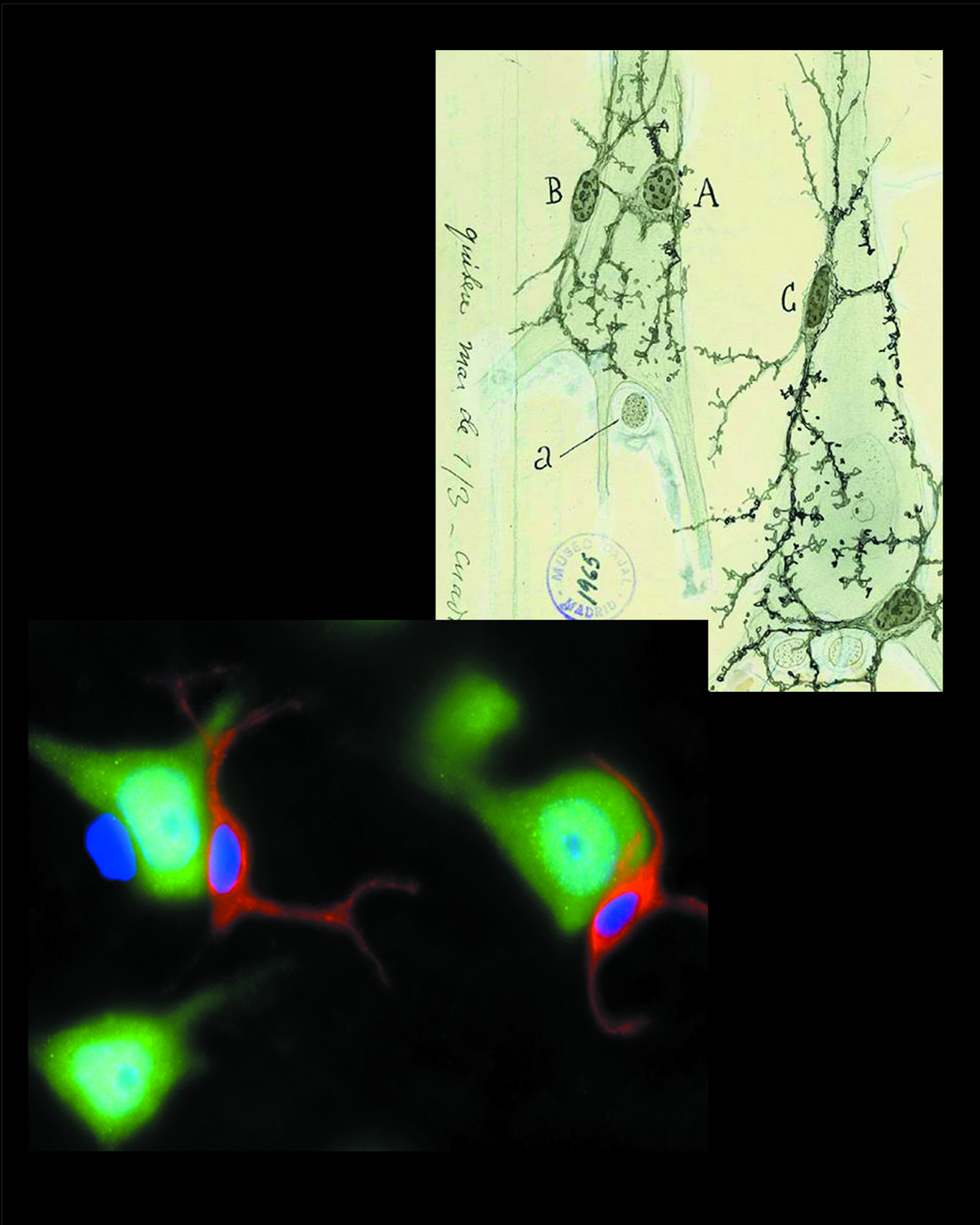
Top right: Perineuronal oligodendrocytes (and/or astrocytes or microglial cells) and their processes abutting neuronal somata as viewed and drawn by Ramon y Cajal (ca 1920). Notice that he detected more than one satellite cell per neuron. Copy of a drawing kept in the Museo Cajal, Madrid, Spain.
Bottom left: Perineuronal oligodendrocytes recognized by polyclonal antibody OTMP (red) in human brain sections. Neurons are stained with NeuN (green) and nuclei with DAPI (blue). The unstained nucleus (blue) adhering to one of the neurons is either an astrocyte or a microglial cell.
Oligodendrocytes can be categorized as precursors, myelin-forming cells and non-myelinating perineuronal cells (pN-OLGs). pN-OLGs have been well characterized morphologically and ultrastructurally, but knowledge about their function remains scanty. It has been proposed that pN-OLGs support neurons and, following injury, transform into myelin-synthesizing cells. We have obtained the gene expression profile of pN-OLGs using a variety of technologies [12]. Our finding revealed that while pN-OLGs belong to the OLG lineage, their phenotype sets them apart from myelinating OLGs in intriguing ways. OLGs carry transcripts of all the major myelin proteins but do not translate them. We speculate that they are kept as reserve in case the cell is called into action after an injury. Indeed, intriguing features suggest that pN-OLGs are poised for myelin repair. First, they possess an autocrine loop consisting of PDGFRa, PDGFRb and their ligand PDGFC that allows them to control their numbers. However, the activation of this loop requires the removal of a CUB domain from PDGFC. Enzymes released during tissue damage would proteolytically remove the CUB segment, allowing PDGFC dimer to bind to and heterodimerize PDGFRa plus PDGFRb, thereby releasing a strong proliferation signal to the cells. Asymmetric cell division would generate one cell to be a pN-OLG and another to become a myelinating OLG. The physiological function of pN-OLGs has yet to be defined. There are claims that they may protect neurons from apoptosis by upregulating lipocalin-prostaglandin D2 synthase (L-PTGDS). Prostaglandin is used therapeutically to slow down the progression of glaucoma. New findings claim a role for pN-OLGs in the development and homeostasis of the prefrontal cortex as their demise leads to cytoarchitectural abnormalities associated with mental disorders. This work shines a new light on pN-OLGs and opens the field to innovative research concerning their putative function as remyelinating cells in gray matter and their role in protecting neurons or in mental disorders.