LSR Fortessa
Traditional benchtop analyzersHome » Benchtop Analyzers » Fortessa
Overview
There are two Fortessas in the CAT Facility. They have a very similar optical bench, and one is equipped with a high-throughput system (plate loader)


Sample Preparation Tips
Accepted Tubes
The LSR Fortessas require a specific 5mL round bottom tube (Falcon #352008). For the HTS a variety of plates are accepted.
Sample Volume
If you are new to flow cytometry, it is safer to resuspend your cells (especially controls) in a larger volume. This will allow you to properly set the instrument settings without running out of sample. As you become more comfortable with the cytometer, you can use more concentrated controls so that they run faster on the instrument.
- 150 µL absolute minimum, 300-500 µL minimum for new users, 150-300 µL for advanced users
- It’s better to bring concentrated samples, once you are at the cytometer you can easily dilute your tubes if they are running to quickly
- In general, run samples at less than 35,000 events/sec
Resources
There is a lot of information available in the Resources Library (located under the Resources menu)! The ones relevant to the Penteon/Quanteon can also be found in the module below.
Software Tutorial
Troubleshooting
Opening the sheath tank
The Instrument is not connecting
The DIVA software should take at most two minutes to connect to the instrument. Beyond that point, power cycle both the instrument and the computer (shut them off completely and reboot).
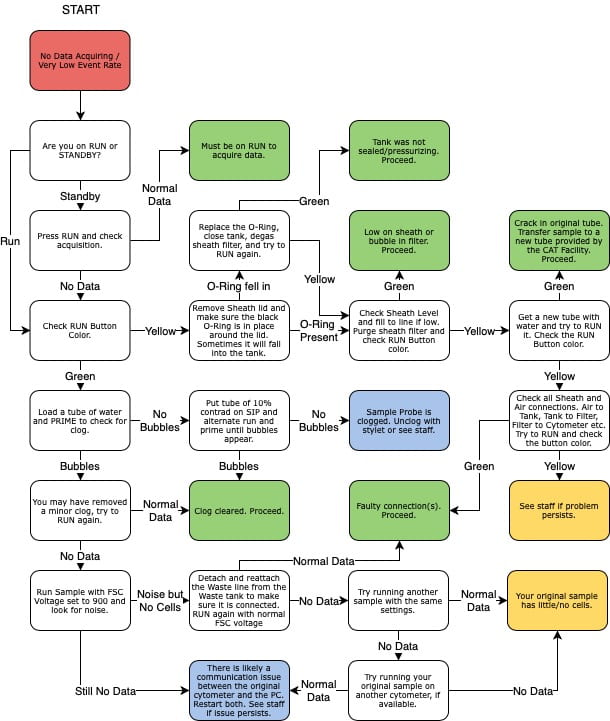
Very few events are showing
A few things to consider:
- Check the Run button - if it has a yellow-ish color, the sample is not being pressurized and is not flowing through the instrument. Common issues include : sheath tank is empty, the sample tube is cracked, the arm is not closing properly. Otherwise, contact the staff for assistance.
- The sample may be clumpy, filter through a 45um mesh to remove obstructing elements;
- The tube support arm may be set too high: the black hockey puck on which the tube sit on the sample injection port (SIP) can be raised or lowered by screwing it clockwise or counter-clock-wise. It may be set too high and blocking access to the sample;
- The adjustment dial on the front panel may be set too low, turn it clock-wise to increase pressure.
The instrument was running on an empty sheath tank
No data is showing
The event rate stays at 0. A few things to consider:
- There could be a communication issue between the instrument and the computer. With a tube of water on the SIP, raise the FSC voltage all the way to 1000. At that level, you should see all sorts of noise on the FSCxSSC graph. If the event rate remains at 0, there is likely a communication issue. Power cycle both the instrument and the computer to restore connection. If the event rate raise to several thousands events/sec, then the connection is fine and the problem elsewhere.
- With a tube of water on the SIP, press the Prime button. Bubbles should be coming out of the SIP during the process. If that is not the case, the SIP is likely clogged. With a tube of Contrad 10% on the SIP, press the Run and Prime buttons, alternating every half second until the clog is dislodged and bubbles are coming out of the SIP.
- If the clog persists, try the advanced unclogging option below.
- Contact the staff if neither these steps are successful.
Unclogging the SIP with a stylet
Please get the approval from the CAT Facility staff before you perform this procedure.
Shutdown and maintenance procedure
- Run 10% Contrad (red taped bottle) on High for 3-5 minutes.
- Run DI H20 (blue taped bottle) on High for 3-5 minutes.
- With the tube of DI H20 still in place, press Prime.
- After ~15 seconds of priming, the cytometer will automatically return to Standby.
- Make sure to leave the instrument in Standby Mode.
- If you are the last user of the day, turn off the cytometer with the green power button.
Data will not upload to the server
- The data should upload automatically every 30 minutes, so be sure that you gave enough time for the transfer to occur.
- Data should b exported to D:/BDExport/FCS. Make sure that you did not export your files in another location - either somewhere else on the disk, or a sub-folder of FCS Export.
- In some case communication gets lost, contact the staff and we will alert our IT manager.
Contact the staff
Email cathelp@bsd.uchicago.edu to reach the CAT Facility staff.
HTS unit maintenance
Coming soon.