Our solution predicts when someone with a pacemaker/ICD is about to experience cardiac arrest, so that a physician can appropriately intervene ahead of time and save the patient from the discomfort and harm of receiving a shock from the pacemaker. We are requesting $250,000 to help fund the upfront clinical trial and proof of concept phase, at which point we will need additional funding to commercialize and scale the product.
Background on the Problem
The global pacemaker market is expected to reach $12.3 billion by 2025, and each year 1 million pacemakers are implanted worldwide. A pacemaker is a small, battery-operated device that is usually placed in the chest to treat arrhythmias, or abnormal heart rhythms. It uses low-energy, electrical pulses to prompt the heart to beat at a normal rate. A similar device called Implantable cardioverter defibrillators (ICDs) can prevent sudden cardiac arrest. There are also new-generation devices that combine both functions.
Although pacemakers and ICDs can deliver lifesaving therapy, they are not always accurate; up to one-third of patients get shocked even when they should not be. This potentially leads to adverse health outcomes, as some trials suggest a strong association between shocks and increased mortality in ICD recipients. Thus, there is a real patient need for a solution that identifies and prevents cardiac arrest even before it happens. Identifying patients at risk can prevent shocks, hospitalizations, and even death, and can also generate quantifiable cost savings: a Stanford study suggests $210 million in Medicare savings could be achieved by introducing this type of technology.
Description of the Solution
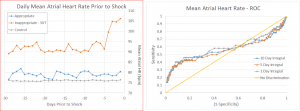
We propose the development of an analytics dashboard for physicians that uses machine-learning algorithms in combination with remote monitoring data collected from the patient’s pacemaker to identify a patient’s risk for cardiac arrest. The algorithm will employ supervised learning as it will initially be trained on de-identified data from patients who have been correctly shocked in the past. This data is already being collected from remote monitoring systems, which collect hundreds of data points each and every minute spanning across 60+ physiologic variables such as heart rate, activity level, fluid backup, and variability in EKG findings. Through an initial pilot study, we found that a number of these variables change in the hours and days leading up to a shock; see the figure below for what a life-threatening cardiac arrest looks like for a device right before it delivers therapy.
Our dashboard would essentially build a layer of analytics on top of the existing ICD logic that will improve the accuracy of the shocks and alert physicians and care teams when certain changes in variables might indicate that a patient is at risk of cardiac arrest. The model will be based on neural networks combined with a support vector model that can relate patients in real time with those that have received a shock in the past. See the figure below for an example dashboard interface, with the tile in the bottom left corner alerting the physician to the patient’s risk level.
Once a patient has been identified to be at risk for impending shock by our dashboard, the care team can provide preventative care. This can include a) medical management (diuresis, antiarrhythmic medications, or hemodynamic monitoring) to prevent further clinical decompensation into cardiac arrest requiring device therapy or b) reprogram the device parameters to prevent inappropriate therapy. Through this “human-in-the-loop” intervention, the algorithm can learn to better risk-stratify patients in need of therapy.
For the second version of our product, we will directly integrate our solution into the medical device itself. By doing so, our solution can provide real-time analysis rather than waiting for home-monitoring data transmission. This vertical integration will be first-in-class and provide an advantage over potential new-entrants who seek to develop a cloud-based solution modeled after our initial solution.
We plan to license our software to medical providers so that they can provide higher-quality care to patients with pacemaker/ICD devices. With a changing reimbursement environment that links financial reimbursement with medical outcomes, we believe that physicians and hospitals will be incentivized to pay an ongoing premium to use the software.
Empirical Demonstration
We will design a prospective randomized control trial that will randomize patients into either a control group or a treatment group. Each patient will be assigned a risk score by our algorithm. The control group will continue to use their pacemaker/ICD as is, while the treatment group will receive additional preventative warnings generated by our algorithm that will alert them to seek immediate help from a physician. We will measure and compare 1) the total number of shocks delivered; 2) the proportion of shocks that are inaccurately delivered; and 3) the number of “interventions” from our algorithm that resulted from a real, elevated measure of patient risk, as ascertained by the physician. A successful outcome for this demonstration would be an overall reduction in the total number of shocks on a risk-adjusted basis (measure 1), a reduction in the “false negative” rate (measure 2), and a low overall “false positive” rate (as extrapolated from measure 3).
Pilot
We conducted a pilot study of over 2,500 patients and found that several key variables change prior to shock. The first graph below shows the elevation in heart rate prior to shock. The second graph on shows the predictive value of this variable in a univariate regression analysis. As you can see, heart rate on its own already seems to be a fairly good predictor of a shock. We then ran a logistic regression on all 60+ variables to identify multivariate correlations. See the figure below for the results of that model.
We plan to expand our algorithm development beyond this initial feasibility study to include analytical methods borrowing from techniques such as “data-smashing” and “hawkes processes” that provide advanced insight into continuously acquired quantitative data streams. The advantages to these methods is that they can infer causal dependence between streams with a relatively small dataset (as compared to a neural network, for example). Beyond the statistical advantages these methods convey, the relatively minimal computational power required provides an added advantage of being incorporated into the pacemaker itself.
After finalizing the first version of the algorithm, we will partner with the University of Chicago Medical Center to launch a pilot prospective research study for patients with pacemakers who receive care at the University. Our algorithm and dashboard will be embedded into the workflow of the arrhythmia care provided to patients with pacemakers to identify potential corrective therapy before an arrhythmia occurs. Upon demonstrating the success of the product at the University of Chicago, we will move from the pilot phase into the full launch of our licensed software to other tertiary referral academic medical centers. We will also explore joint-development with device manufacturers as they have a strategic interest in improving their product through advanced analytics.