by Elise Wachspress
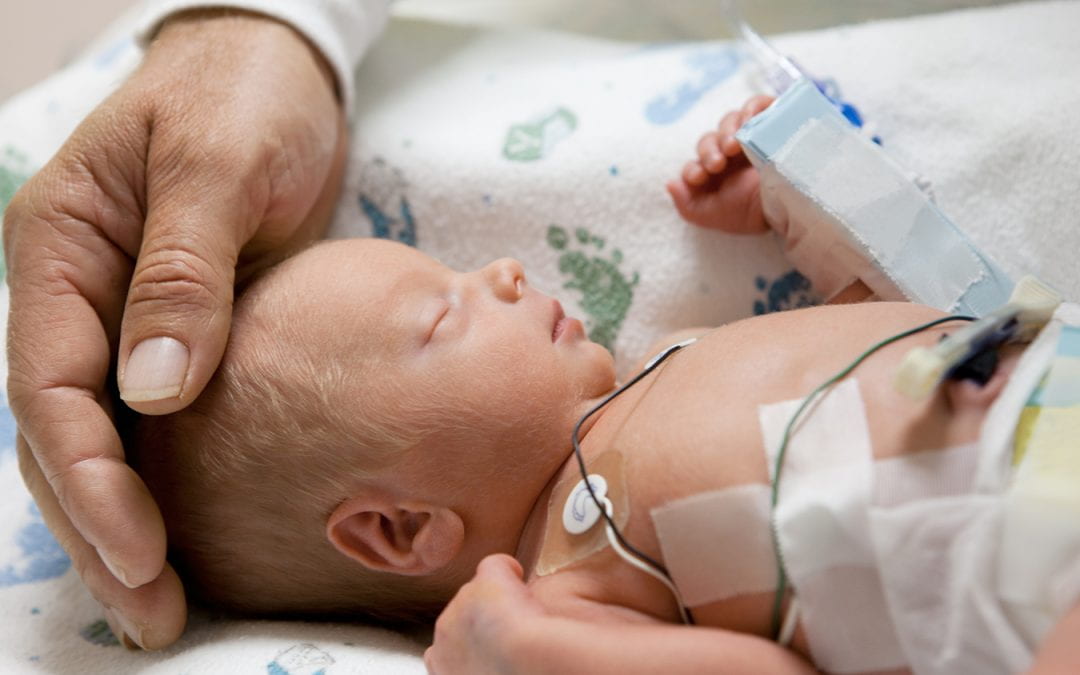
Neonatologist Erika Claud, MD, has long been thoughtful about the developmental effects of the treatments she and others use to save the lives of tiny premature babies. With the help of a multi-million dollar investment from the National Institutes of Health, Claud and collaborators across the nation have been looking for ways to improve the outcomes of these babies, not just in the hospital but at home and in school, as they grow to catch up with their full-term age-mates.
Because it is common practice to treat vulnerable preemies with antibiotics, Claud was especially curious about how this approach affected the microbiome which all newborns must establish in their guts to enjoy a healthy life. Of course, one of the first benchmarks of progress for these tiny humans is weight gain, and Claud’s team has already identified microbial factors that can influence how fast these compromised babies grow.
Just as important to address—perhaps even more important—are the longer-term effects of prematurity and low birth weight, including cognitive and behavioral outcomes. Claud suspected (and the NIH found her argument compelling) that the composition of the pre-term baby’s microbiome, shaped by a hospital environment with constant instrumentation, frequent antibiotic use, multiple caregivers, and less consistent access to the microbiome of his/her own parents, might also affect brain development.
So she and her team set out to find how microbes in the gut affect the composition of fatty acids in the brain and the mechanisms of neuro-inflammation, which alters how the synaptic network, the critical electrochemical connections between nerve cells, is organized. Claud knew that neuro-inflammation could not only cause neonatal brain injuries, including cerebral palsy, but could lead to later motor and cognitive deficits, autism, even schizophrenia.
Of course, Claud and her team weren’t about to experiment on the preemies in their care, so they took microbiota from preemies experiencing poor growth and used those samples to colonize the pups of newborn mice. They also took samples from babies who were thriving and colonized other newborn pups with their bacteria, as controls.
While it appeared that differences in the microbiome did not change the fatty acid composition in the brain, the team found differences in the neurotransmission pathways. Mice with microbiota from the infants with poor postnatal growth had lower levels of specific growth factors, both circulating in their blood and in their brains. They also had higher levels of neuro-inflammation, associated with compromised synaptic organization in developing brains.
In the paper documenting their results, Claud and her team note that the first days and weeks of life constitute a critical period: “Our study characterized the effect of the early microbiome, from less than two weeks of life in preterm infants, on neuronal and myelination development in the early postnatal ages, providing direct evidence that there is a critical window in early life during which microbiota can influence brain development.” And the journal’s readers were fascinated; the editors of Scientific Reports noted that the paper was one of their most-read neuroscience stories last year.
Claud is now working with Nobel laureate and Chicago faculty member James Heckman in his long-term effort to connect cross-disciplinary experts to advance innovative approaches to human capital development research and improve individual opportunity worldwide. See Claud talk about how her research in neonatal intensive care is helping to advance the cause of Human Capital and Economic Opportunity. In this way, scientists affiliated with the Duchossois Family Institute will be improving the lives of people for decades into the future.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office