
Jan 22, 2020 | Microbiome, Neuroscience
by Helen Robertson
What is your biggest concern about growing old? A decline in physical fitness? A loss of independence? Or perhaps it’s the fear that your mental fitness might start to lose its edge?
For the 50 million people worldwide living with dementia, that last scenario is a reality. A dementia diagnosis comes with big personal, social, and financial consequences: the cost of care for someone living with dementia is reportedly higher than that of both heart disease and cancer combined.
The most common cause of dementia in the US is Alzheimer’s disease. Although its symptoms are well known—cognitive decline, neuroinflammation, and the tell-tale formation of amyloid plaques, the hard aggregation of proteins between nerve cells in the brain—the precise cause remains unknown, and there is no current cure.
As the world population continues to age, dementia is increasing. The need to uncouple its complex biological processes is urgent.
Sangram Sisodia, PhD, has spent the past three decades investigating just that. But in recent years his Alzheimer’s research has taken an exciting and unexpected focus: the gut.
Thanks to recent findings, many of them by research teams at UChicago, we have learned that the bacteria living in our guts can affect many aspects of health. Normally, our gut microbiome contributes to everyday wellbeing and immunity. But just like any other community, the composition of our microbiome can fluctuate on a near-daily basis. And when a shift in balance occurs, things can go awry.
Our intestinal microbes have a particular influence on immunity and neurological function, both important factors in Alzheimer’s. Those with the disease have also been found to experience a change in the character of their gut microbiome.
That’s where Sisodia stepped in.
Over the past few years, his team has been using mouse models of Alzheimer’s to understand how the composition of our gut microbiome might influence neurological inflammation caused by certain immune cells. They thought this inflammation could contribute to both the protein deposition and neurodegeneration in Alzheimer’s.
Sisodia’s research has already generated some interesting findings. His studies, published in Scientific Reports and the Journal of Experimental Medicine, showed that the long-term treatment of mice with broad-spectrum antibiotics reduced neuroinflammation and slowed the growth of amyloid plaques.
After treatment, the mice also showed significant changes in the composition of their gut microbial communities. Some types of bacteria completely disappeared; others multiplied—suggesting bacterial diversity in the gut plays a role in the immune response during disease progression.
But only for male mice. In females, antibiotics actually increased the inflammatory response, with no change in brain plaques. With Alzheimer’s more prevalent in women than men, this gender difference in immune response clearly warrants more study.
Myles Minter, postdoctoral scholar in Sisodia’s lab who is now a research analyst at William Blair, wondered what might happen if one could prevent Alzheimer’s by treating it early—really early. He gave two-week-old mice pups antibiotics for just one week—which left lifelong effects on both their gut microbiome and amyloid plaque formation.
But this is no simple solution. UChicago neonatologist Erika Claud has shown how changes to the microbiome of premature babies can have a negative impact on neurological development, and Eugene Chang found mouse pups whose mothers were treated with antibiotics were more likely to develop inflammatory bowel disease.
Constantly treating individuals with antibiotics is not a realistic scenario, even for those with genetic predisposition for Alzheimer’s, but Sisodia is keen to investigate further. He has recently been awarded a grant of over $2,000,000 to continue his research into Alzheimer’s disease and immunity. The money comes from the Good Ventures project, involving Massachusetts General Hospital, University of Southern California, Northwestern University and Washington University, with total funding over $10,000,000. The hope is this collaboration can uncover mechanisms at play between our microbiome, our immune system, and Alzheimer’s disease.
This is just another example of how the microbiome offers keys for exploring new preventative and treatment approaches for healthy longevity. As Bette Davis once suggested, “Old age ain’t no place for sissies,” but maybe someday losing our mental fitness may not top our list of concerns about aging.
Helen Robertson is a postdoctoral scholar in Molecular Evolutionary Biology at the University of Chicago, with a keen interest in science communication and science in society.
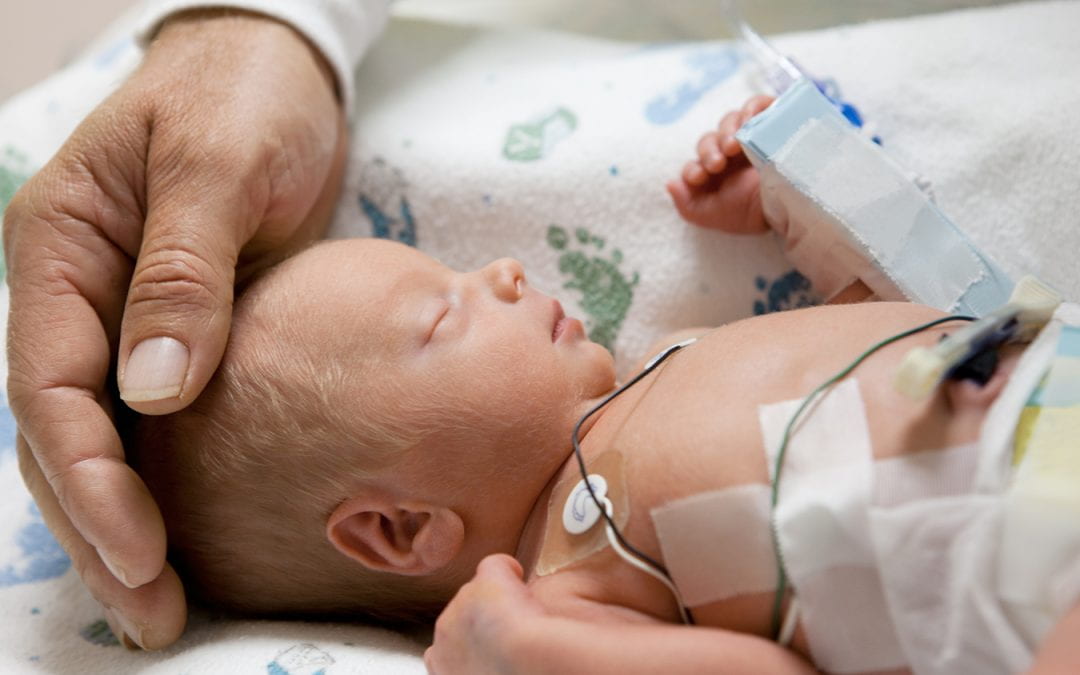
Aug 9, 2019 | Microbiome
by Elise Wachspress
Neonatologist Erika Claud, MD, has long been thoughtful about the developmental effects of the treatments she and others use to save the lives of tiny premature babies. With the help of a multi-million dollar investment from the National Institutes of Health, Claud and collaborators across the nation have been looking for ways to improve the outcomes of these babies, not just in the hospital but at home and in school, as they grow to catch up with their full-term age-mates.
Because it is common practice to treat vulnerable preemies with antibiotics, Claud was especially curious about how this approach affected the microbiome which all newborns must establish in their guts to enjoy a healthy life. Of course, one of the first benchmarks of progress for these tiny humans is weight gain, and Claud’s team has already identified microbial factors that can influence how fast these compromised babies grow.
Just as important to address—perhaps even more important—are the longer-term effects of prematurity and low birth weight, including cognitive and behavioral outcomes. Claud suspected (and the NIH found her argument compelling) that the composition of the pre-term baby’s microbiome, shaped by a hospital environment with constant instrumentation, frequent antibiotic use, multiple caregivers, and less consistent access to the microbiome of his/her own parents, might also affect brain development.
So she and her team set out to find how microbes in the gut affect the composition of fatty acids in the brain and the mechanisms of neuro-inflammation, which alters how the synaptic network, the critical electrochemical connections between nerve cells, is organized. Claud knew that neuro-inflammation could not only cause neonatal brain injuries, including cerebral palsy, but could lead to later motor and cognitive deficits, autism, even schizophrenia.
Of course, Claud and her team weren’t about to experiment on the preemies in their care, so they took microbiota from preemies experiencing poor growth and used those samples to colonize the pups of newborn mice. They also took samples from babies who were thriving and colonized other newborn pups with their bacteria, as controls.
While it appeared that differences in the microbiome did not change the fatty acid composition in the brain, the team found differences in the neurotransmission pathways. Mice with microbiota from the infants with poor postnatal growth had lower levels of specific growth factors, both circulating in their blood and in their brains. They also had higher levels of neuro-inflammation, associated with compromised synaptic organization in developing brains.
In the paper documenting their results, Claud and her team note that the first days and weeks of life constitute a critical period: “Our study characterized the effect of the early microbiome, from less than two weeks of life in preterm infants, on neuronal and myelination development in the early postnatal ages, providing direct evidence that there is a critical window in early life during which microbiota can influence brain development.” And the journal’s readers were fascinated; the editors of Scientific Reports noted that the paper was one of their most-read neuroscience stories last year.
Claud is now working with Nobel laureate and Chicago faculty member James Heckman in his long-term effort to connect cross-disciplinary experts to advance innovative approaches to human capital development research and improve individual opportunity worldwide. See Claud talk about how her research in neonatal intensive care is helping to advance the cause of Human Capital and Economic Opportunity. In this way, scientists affiliated with the Duchossois Family Institute will be improving the lives of people for decades into the future.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office

Apr 1, 2019 | Microbiome
by Folabomi Oladosu, PhD
Post-doctoral researcher specializing in pain and women’s health at NorthShore University HealthSystem
Holding a newborn for the first time simultaneously conveys the fragility of life and the enormous responsibility of ensuring this tiny person’s progression to adulthood. All the coos, gurgling, and late night cries are important steps in a newborn’s developmental process. These behavioral cues let us know that this baby is growing up exactly as she should.
Unfortunately, newborns born preterm—earlier than expected—are more likely to have developmental delays. Scientists are looking for ways to help reduce or eliminate some of these delays, and recent research indicates that the gut microbiome may be a key factor.
Before emerging into our messy world, a baby lives in an almost microbe-free environment. It’s during birth that most newborns are introduced to a great variety of helpful microorganisms, especially from her mother. The infant meets additional friendly bacteria as she nurses from her mother’s breast. These microbial partners begin teaching the baby’s immune system how to recognize friends—bacterial or chemical—and distinguish them from bad actors in the same way parents teach their children the difference between Aunt Mary and “stranger danger.”
Along with the immune-boosting proteins delivered via breast milk, these helpful bacteria give the newborn’s immune system the time and experience to learn and mature. In fact, most healthy, full-term infants show a pattern of progression where their gut microbiome evolves to look pretty much like an adult’s by about age two and a half.
But preterm newborns are different in several ways. They are more likely to be delivered via Cesarean section, a route that exposes infants to far fewer microbial allies. And because of their size and shortened development in utero, preterm infants usually need to stay in the hospital longer than most newborns, with reduced access to mom’s breast and the helpful microbes and immune-boosting proteins that come along with it. This means the baby’s immature immune system get less expert training and is thus more susceptible to infections.
To prevent these infections, preterm infants are often given antibiotics, a course that can set up additional problems. Antibiotics can kill bad guys but also destabilize the microbial community developing in the gut, interfering with the immune training that helps the baby thrive in the long run. It is thus important to understand the tradeoffs in using antibiotics and choose the best treatments for the long-term health of each little person.
Alyson Yee, a student in UChicago’s Medical Scientist Training Program (an integrated MD/PhD program) and then a member of the lab of Jack Gilbert (now at the University of California San Diego), wanted to consider that issue. “We think there’s a critical window in early life to train the immune system by being exposed to the right microbes,” said Yee. “For example, kids born by Caesarean section, with less exposure to microbes from their mother’s gut and vagina, have a higher lifelong risk for allergy and asthma. Childhood exposures to the environment, especially pets, can also change the microbiome and the immune system. This is why it’s so important to study the microbiome in infancy.”
Of course, the most sensitive infants are the tiniest. So Yee set out to analyze the gut microbiome of infants with very low birth weights by determining how their microbial profiles differed from full-term babies then examining how those differences affected weight and length gain during the first six weeks after birth. For a subset of those babies, she and her team were able to retest the children at two and four years of age.
Her team looked at two specific characteristics of the gut microbiome: the overall diversity, a sign of microbial health, and the rate of microbial turnover, a key measure of progress to microbial maturity.
At six weeks, the team found that very-low-birth-weight babies with greater microbial diversity who received a greater percentage of milk from their mother’s breast were gaining weight somewhat more quickly. But they also found that antibiotic exposure encouraged an abundance of proteobacteria, a kind of resistant microorganism commonly found in hospitals that can become opportunistic pathogens. Together with two other specific types of bacteria found in those on antibiotics, proteobacteria were correlated with poor weight and length gains. In contrast, infants with increased microbial turnover—including those delivered by C-section—tended to have fewer of these bacteria and grew faster during their hospital stay.
The best news is that microbial diversity in most of these very-low-birth-weight babies increased significantly over time. By age four, the baby’s microbial profile was very similar to that of her mom’s, proving yet again the resilience of the human organism.
It’s interesting to note that researchers have found similarities between the preemie microbiome and that of full-term infants who are undernourished—suggesting that therapies developed for one group may be helpful for the other.
Given the relatively small number of very-low-birth-weight babies they followed at six weeks (83) and at four years (25), the researchers suggest continuing expanded studies. UChicago neonatologist Erika Claud is already leading a major study that will follow of thousands of premature babies over five years to understand how the development of the microbiome affects childhood development and even school readiness. Stay tuned for more clarity on how we can promote the greatest health for our littlest fellow humans.






