by Roma Shah
In this COVID-19 pandemic, the need for virus research has never been greater. People are dying, and right now there seems no effective way to stop it. In New York City, the situation has been overwhelming. Experts suggest this tragedy isn’t over; even if this first wave improves, the coronavirus may reappear, and by then we must understand its mechanisms, its manner of infection, and somehow create a solution.
Michaela Gack, PhD specializes in virology and immunology research, focusing on interactions between viruses and the immune systems of the hosts they infect. She breaks down this pandemic into three component areas: what it is, how people get it, and some ways researchers are looking to intervene.
COVID-19 is caused by a virus that belongs to a larger family of coronaviruses, including those that caused severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), which emerged in 2003 and 2012 respectively. But the COVID-19 virus is a completely new version, and virologists have much to learn to stop this pandemic.
How do people get the disease? The virus, also called SARS-CoV-2, has proven to be highly transmissible, spread easily through the community, and we are just beginning to learn how. Complicating this problem is that a number of people who don’t show symptoms of infection apparently seem able to unknowingly spread the disease.
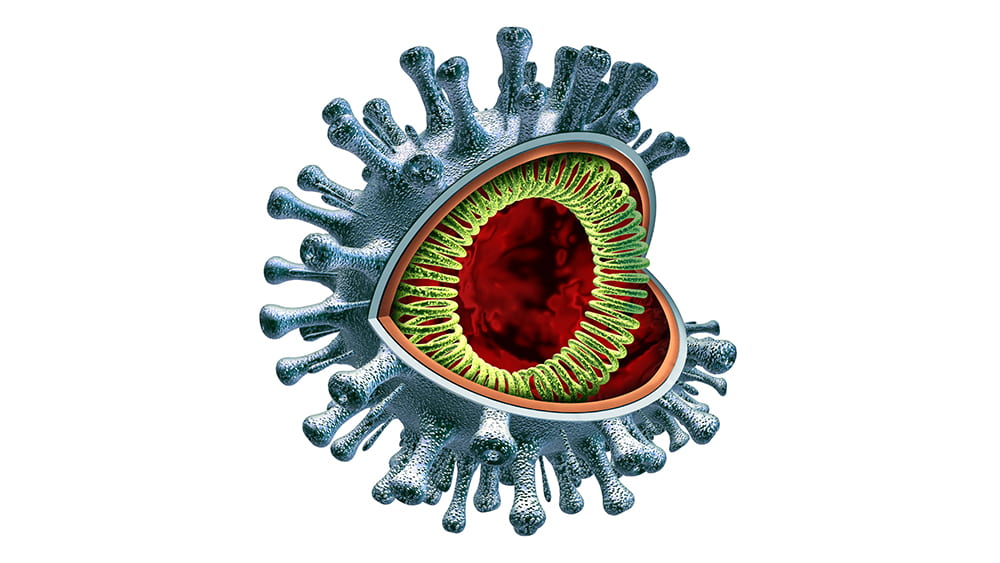
Although the virus—a very large piece of RNA encased in a protein capsule topped with a crown, or “corona” of spikes— is not “alive” by itself, it is extremely effective in using live cells for energy and spare parts to create many new versions of itself. By working to understand how it operates, Gack and virologists around the country hope to find ways—perhaps multiple ways—to intervene. They are considering several main opportunities.
The first is when the virus first hooks onto a human cell. It does so by attaching to an enzyme called ACE2 which is on the outer surfaces of the cells in the lungs and other organs. Imagine a locked door, where the virus must have the exact key to get into the cell. If scientists can find a way to jam that lock so the key can’t fit, then the cell will stay safely free of the virus.
One approach is “spike-binding”–creating an antibody that will clamp onto the virus’ “keys” and keep them from inserting into the lock. This is how a vaccine works, priming the immune system to make substances that lock onto the keys and keep them from spiking into the cell. But until scientists can develop that vaccine, we can gum up the keys by providing antibodies from patients who have successfully fought off the disease—what scientists call “passive immunity,” until we generate the vaccines to that will help patients to make their own.
Once the virus gains entry into the cell, it must next dissolve its protein coating and release its RNA payload. This is the point where some have suggested hydroxychloroquine might work. The drug, used to treat malaria and lupus, decreases the acidity inside cellular vesicles, working somewhat like a knot in a scarf, to keep the viral coating intact.
Once the RNA is released, it starts replicating uncontrollably, in an interesting way: it uses the building blocks inside the cell to generate long strands of RNA. An enzyme encoded by the virus called polymerase facilitates this process, adding those building blocks along until the chain is complete. Some of these RNA molecules are used to make constituent viral proteins, up to a million of them in each cell.
This is where the drug remdesivir has been hypothesized to work. Remdesivir very closely resembles one of the four main building blocks of RNA. So, when the polymerase is building the chain and looking for the RNA components, it could accidentally grab remdesivir instead, and the replication process falls apart. Think of using screws to put furniture together, where two look very similar. Pick the “wrong” one and the whole desk falls apart.
One more avenue for intervention is in editing the RNA molecules of the virus once they are in the cells. Cellular RNAs contain more than a hundred fifty important modifications at thousands of sites, some with critical regulatory roles analogous to those of protein and DNA modifications. UChicago researchers Chuan He and Tao Pan are world leaders in RNA modifications, and they are now working on ways to deactivate the RNA production that takes over the cellular machinery.
This COVID-19 pandemic, all its unknowns, and all these possible avenues for treatment remind us once again of the important role researchers play even in the most emergent situations, searching for results that can benefit our society as soon as possible.
Roma Shah is a second-year undergraduate studying neuroscience and public policy.