
Aug 17, 2020 | COVID-19, Immunology
by Elise Wachspress
All systems depend on moving information. Whether you are running a household, a company, or a country, relaying knowledge is critical.
As institutions grow in size and complexity, new forms of communications become ever more essential. Drums, yodeling, and semaphores have given way to cell towers and the Internet to relay information around our big, curved planet. Complicated systems need network nodes and signaling that transmit knowledge quickly, reliably, and accurately.
Our own bodies also depend on information transfer as well, and thanks to evolution, our biological systems are remarkably well-honed. Millennia of natural selection have left us with internal signaling tuned precisely to deliver the intel our cells need to keep us healthy and functioning.
But, in every system, some new problem always comes along.
This year, it’s COVID. It invades our bodies, commandeers cellular machinery, causes tissue damage, and, most dangerously, hijacks our internal signaling system, invoking a storm of immune agents that fails to kill the virus and instead kills the host—us.
The most straightforward intervention strategy is just to neutralize the interloper: develop a vaccine or a drug that kills off the coronavirus and go back to our normal lives. And many labs around the word, including some at UChicago, are working on that.
But in a situation as potentially cataclysmic as this pandemic, it behooves us to work in tandem on multiple strategies. What if we took a host-centric approach and figured out how to regain control of our internal signaling system so the virus loses the power to cause serious damage?
A huge group of UChicago scientists and clinicians, in areas from cancer to chemistry to pulmonology and more, are already working together on this, and investigators from Northwestern and the University of Illinois at Chicago have now joined them. They are using their vast collective knowledge of cell signaling pathways—the movement of information from one internal “cell tower” to another—and molecular therapies to figure out how to keep the immune system on track after coronavirus infection.
The team’s is a three-step approach. First, survey compounds known to be active at various points in cell signaling, identify those with antiviral potency, and test them on lung cancer cells. This will help them identify “nodes” where the hijacking is happening so they can shore up these weak links in the signaling pathway. This is possible thanks to some fairly new technological approaches known collectively as “omics”: transcriptomics, proteomics, metabolomics. Together, these can show how the cell is responding to environmental stresses: which parts of the cell’s genome are activated, what proteins it’s making (important because viruses need LOTS of proteins to replicate), and what other byproducts are formed.
The next step is to see how these “omics” compare with those in cells actually infected with the coronavirus. To do so, researchers will take cells from healthy patients into the lab and inject them with the virus. The differences and similarities they see between the “omics” of the cancer cells and the COVID cells will tell them a lot about how to proceed. These infected cells will also serve as a substrate for testing the compounds identified earlier, thus sussing out the most promising candidates for drug development.
These first two projects are only possible because the team has access to UChicago’s Howard T. Ricketts Laboratory, one of 13 regional biocontainment facilities in the US, located at Argonne National Laboratory, also managed by UChicago. (As you might imagine, the demand for services at Ricketts has multiplied exponentially over the past few months.)
Once the team has identified compounds active in calming relevant parts of the signaling pathways, they will start testing various drug cocktails in live organisms, at first mouse models. The team will see which can tamp down the “cytokine storm,” the signature, out-of-control immune response to COVID-19 that damages the lungs, and, we are learning, often cardiac and brain tissue as well.
The team started the research with a seed grant from the University’s Big Ideas Generator program, set up specifically for the kind of promising, “out-of-the-box” ideas rarely funded by the government. Now most of the team are borrowing resources from other projects in their individual laboratories to continue the momentum. The landscape is full of researchers desperate for support to see if their approaches can help stop this pandemic, and even once grants are won, getting the money rolled out is slow. So Marsha Rosner, PhD, the leader of this strike force, is hoping for early help from a philanthropic “angel.”
What is unusual about this team—its size, coherent integration, access to unusual laboratory resources, and the infrastructure to make eventual clinical testing possible—suggests they have a better shot at success than most.
The payoffs may be large. Because they are looking closely at the host—our own cells—rather than this specific virus, what this team learns may eventually help protect us from other respiratory viruses. So when the next new problem comes along, we’ll have a better understanding of our immune system and be much better prepared to keep the deaths from mounting.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office.

Aug 11, 2020 | Celiac, Immunology
by Elise Wachspress
While we often think of our immune cells as fighting the good fight against bacteria, viruses, and our own rogue cells (as in cancer), the system is sometimes reacting to specific dietary antigens, chemical toxins and even healthy tissue it mistakenly perceives to be dangerous microbes. We’ve long called bread “the staff of life,” but there are some people whose immune systems think of it more like an incitement to all-out war.
For those with celiac disease, the immune army resident in the gut sets their weapons against gluten, a protein in the seeds of wheat and other grains. In the celiac gut, the “heat of battle” plays out as serious inflammation of the villi, the “little fingers” of the small intestine that facilitate nutrient absorption. And because wheat is such a staple of our diets—far more than just the main ingredient in our daily bread and beer, but also a thickener in soy sauce, imitation meats, ice cream, ketchup, and even cosmetics and hair products—the immune cells of people with celiac are pretty much fighting this battle all the time.
Luckily, there are now good tests for celiac disease, and, in most who follow a gluten-free diet, the immune system activation stops, and the villi can heal.
This research, led by Bana Jabri, MD, PhD, and postdoctoral fellow Toufic Mayassi, PhD, now at Harvard/MIT’s Broad Institute, along with a host of scientists (immunologists, gastroenterologists, chemists, geneticists, and molecular biologists) from Chicago, Wales, the Netherlands, and Australia, suggests that patients with celiac disease may at higher risk of cancer and infections, especially if they were diagnosed as adults.
This research also encourages families of patients with celiac to proactively seek screening for the disease. Recent research by the Mayo Clinicfound that 44 percent of close relatives of celiac patients also test positive for the disease, even though they show no or atypical symptoms. The UChicago team findings accentuate the importance of testing close relative of celiac patients—to preserve the long-term immune flexibility of their gut cells.
This research also suggests more universal vulnerabilities for all of us: that constant inflammation can permanently reconfigure our immune cell population and increase our disease risk, providing ample reason for an anti-inflammatory diet of fruits, vegetables, nuts, fatty fish, and red wine—besides the fact that these are just plain delicious.
The esteemed journal Cell found this research so compellingly important that they invited Jabri and Mayassi to submit a video explanation—just more research coming out of the Jabri lab that identifies her as one of the world’s leading experts of both on celiac disease and the immune response in general.

Jul 10, 2020 | COVID-19, Immunology
by Elise Wachspress with Peter Wang
Before COVID, before the Spanish flu, there was another highly infectious pulmonary disease that also changed entire countries and societies.
Tuberculosis, however, has been with us since antiquity. Like COVID, TB travels from person to person in droplets or aerosols. Caused by a mycobacterium, the disease was the most common cause of death in the nineteenth century. Populations who lived or worked in very close quarters—like migrants traveling in the holds of ships or poor people stacked in urban tenements—were the most vulnerable. But “consumption” was also somehow considered “romantic,” a wasting disease that also felled many writers and artists, the young John Keats famously among them. In fact, Edvard Munch’s “Sick Child,” above, documents his own sister dying of TB at age 15.
The advent of antibiotics made a big impact in curing the disease. But the mycobacteria that cause TB have evolved resistance, and poverty is still endemic around the world. The sad fact is that the disease still kills a million and a half people every year. That’s why the Bill and Melinda Gates Foundation is interested in TB research. One of the scientists whose work they support is UChicago’s Luis Barreiro, PhD.
Before antibiotics, French scientists Albert Calmette and Camille Guerin had already developed a vaccine against TB. In a way, it was similar to how the smallpox vaccine was developed from the much-less-virulent cowpox: Calmette and Guerin developed a vaccine for TB from a much less lethal bacterium also found in cows.
But BCG works unlike other vaccines. Most train the adaptive immune system, the specialized, “intelligent” commandoes that home in on very specific molecular targets. BCG also seems to affect the body’s innate immune system, a generalized assembly of cellular fighters once thought to be pretty untrainable. One clue is that BCG given in infancy or early childhood seems to reduce vulnerability to a whole range of diseases, not just TB.
And people now suspect that COVID-19 may be one of these.
Barreiro was working on TB and BCG long before the coronavirus reared its ugly head. He is developing the evidence that BCG ends up in the bone marrow, where it changes the epigenetics—the chemical “decorations” that hang off the basic structures of DNA and RNA—of the stem cells developing there. It creates something like a boot camp where newly emerging macrophages—the infantry grunts that rush to the front as part of the innate immune system—become better fighters against multiple diseases at once.
So the Gates Foundation is funding Barreiro’s research to understand whether this mechanism works in humans as it does in mouse models. In low-to-middle-income countries with high rates of TB and other infections, BCG is still given in infancy or early childhood and is known to reduce neonatal mortality for all causes. The Gates Foundation, focused on reducing TB in those countries, wants to understand how BCG works and if that knowledge can help scientists develop an even better vaccine.
One of Barreiro’s special talents in this quest is his abilities with “single-cell” technologies, because you can’t really characterize how different macrophages are working by looking at an “average” of their epigenetics and the “average” interactions they have with intruders. Barreiro uses automated processes that can look at individual cells—a lot of them, very quickly—to generate a fine-grained picture of how BCG vaccination fosters epigenetic variations in certain macrophages that allow these cells to fight many diseases.
Maybe even COVID.
As several recent journals have reported, there is growing evidence that countries with national BCG childhood vaccination programs have a much lower incidence of severe disease and death from COVID. So by developing an understanding of how BCG works, Barreiro and his team may generate important clues in protecting people from our latest scourge.
Science can’t, shouldn’t, won’t sit still and wait to address the next crisis. The work on TB (done centuries ago) and single-cell technologies (perfected over the past few years) may provide the answers to a crisis we didn’t even know we’d have to face.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office. Peter Wang is a second-year undergraduate student in The College.

Jun 8, 2020 | Bioinformatics, Immunology, Microbiome
by Elise Wachspress
If you hang out with cutting edge scientists, you might hear or see the word (the suffix? a crossword puzzle answer?) “omics.” What are omics?
More than likely you‘ve heard of genomics, the study of the structure, function, evolution, and mapping of genomes, the collection of all the DNA in each organism.
And perhaps you’ve heard of transcriptomics, the study of all the ways an organism’s DNA is “transcribed,” or written into smaller molecules, the RNAs. While the DNA provides the basic, relatively unchangeable blueprints, environmental needs in the cell prompt the activation of specific genes. It’s like the highway engineer who, using her part of the blueprint, stages and directs the construction of one of the on-ramps. She’s working in concert with the larger plan, but somewhat separately from those directing other parts of the project.
Then there’s proteomics, the study of the structure and function of all the proteins that carry out the business in the cells. If the genome is a blueprint, and the engineering crew the RNA, the proteins are the molecular machines and building blocks—the backhoes, drills, and concrete—used to carry out the design.
The newest of the ‘omics fields is metabolomics: the study of all the chemical outputs of our cells and every microorganism that lives in and on us. These molecules, taken together, reflect the entire, functioning system, like how the cars and trucks using the highway are moving and thus creating new capital for society. Metabolomics is something like Here or Google maps, measuring important indicators of how the system is performing at both the street and system level. In a biological system, a genome can tell you what is possible, but the metabolome tells you what is actually happening.
But these readouts are more complex and critically useful: how a particular drug is working, how our immune system is responding, how microbes inside us are contributing to our health or modifying their environment to outcompete others, or even how our brains are prompting our bodies to act, and vice versa. New metabolomics technologies can help scientists non-invasively identify disease biomarkers, discover microbial products that can become new drugs, and identify the safest, most efficient ways to maintain health.
Among the many important resources the Duchossois Family Institute (DFI) is developing at UChicago is a facility that specializes in metabolomics. Led by Jean-Luc Chaubard, the DFI Host-Microbe Metabolomics Facility will feature state-of-the-art mass spectrometry, a powerful analytical technique that can be used to detail the profile of complex mixtures, whether solid, liquid, or gas. With this and other advanced instrumentation, DFI scientists will be able to understand the balance of molecules in blood, plasma, saliva, fecal, and even tissue samples, as well as in the waste products left behind when microbes are cultured (grown) outside the body.
Chaubard and his group will be looking at many things: from neurotransmitters and amino acids to bile acids and short-chain fatty acids, recently identified as critical to a healthy immune system. They will use large chemical libraries to create specialized “panels” that can profile multiple metabolites simultaneously. With these capabilities on campus, individual investigators will have ready access to new assays tailored specifically to their work.
The Metabolomics Facility will also help DFI investigators hone experimental design, decide when and how best to collect and store samples, and prepare those samples for testing. Importantly, they will also help in the data analysis that is critical in massive data-collection regimens like mass spec.
Chaubard, with a background both in academia (at Memorial Sloan Kettering and Caltech) and business (as founding director of the Molecular Discovery Lab at Modern Meadow, in New Jersey) is up for the challenge. An entrepreneur at heart, he is excited to be launching a resource that will set up UChicago as a leader in studying the convergence of immunology, the microbiome, and human health: “Here at UChicago, I get to work with some of the best scientists and doctors in the world, translating their work into practical applications that range from mechanistic understanding of human biology to early disease detection and discovery of novel drugs. It’s an honor to have this opportunity to improve the human condition.”
The Metabolomics Center is just one of the new platform resources made possible by a $100 million gift from The Duchossois Family Foundation and Craig and Janet Duchossois. We will bring you descriptions of several more in the weeks to come.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office.

Jun 1, 2020 | Immunology
by Peter Wang and Elise Wachspress
How does COVID-19 pervert the very mechanism designed to destroy it?
Our bodies contain magnificent networks of cells, signals, and interactions that serve as sentries against harmful bacteria and viruses. But in the case of the coronavirus, it seems the most lethal blow sometimes comes from the immune system itself.
When a virus or bacterium—or sometimes even an unrecognized substance—enters the cells of the body, our immune systems send out small proteins to signal “get the war on these aliens started.” These small proteins, called cytokines, then attach to the outside of the cells and rev up inflammation, heating up the environment in an attempt to rid the body of the offenders.
In the case of this novel coronavirus, the response to the interlopers can quickly escalate, with more and more cytokines produced—a “cytokine storm.” It’s kind of like sending thousands of soldiers and tanks trampling across a landscape when the actual enemy is a guerilla cadre hiding safely underground: the potential result is a lot of damage done without ever defanging the real enemy.
For decades, Thomas Gajewski, MD, PhD, has led investigations of how to help the body fine-tune immune responses to cancer. In the coronavirus emergency that has commandeered all our lives, Gajewski is now working to understand the mechanisms of the immune response against SARS-CoV-2 in an effort to tamp down what may be the most dangerous element of this disease.
Within the first few weeks of the coronavirus pandemic in Chicago, Gajewski had put together a team of 40 investigators—from cancer clinicians to microbiome specialists to molecular engineers—to focus on detailing the mechanisms when the immune response to COVID-19 goes haywire. They organized a clinical study of 600 people—500 confirmed to have the disease and 100 healthy controls—and started measuring lots of parameters on each: the patients’ DNA sequences, blood cell composition, cytokines in the serum, antibodies and T cells against the virus, airway microbiota. They were looking for any relevant factor that shaped the immune response and potentially led to severe disease—the kind requiring intensive care unit support—versus mild disease that resolved spontaneously.
The goal: to understand which of these parameters was important in causing favorable or unfavorable outcome, and how clinicians could use these biological markers to decide the best treatment for each patient.
Already, Gajewski and his team had noticed that the lungs of some patients seemed to be full of macrophages, large white blood cells that protect places where our tissues meet the outside environment. And these macrophages seem to put out high levels of a specific cytokine known as interleukin-6 (IL-6), often implicated in severe cytokine storm.
Gajewski was well-positioned to launch this effort for multiple reasons. As a cancer immunologist, he has been studying how to increase or tamp down immune responses to disease for decades; he published his first papers on the interleukins nearly thirty years ago. To advance insights in cancer during decades of medical practice, he has relentlessly collected and carefully assessed as many patient samples as possible; these include samples less commonly studied, like stool—where he and his lab can identify the presence of the gut microbes that affect the immune response. And serendipitously, Jonathan Trujillo, MD, PhD, a fellow in Gajewski’s lab supported by Ruth and Elliot Sigal, had studied cytokines in coronaviruses earlier in his training, providing a direct expert on the team.
Gajewski and his team have already developed assays that reliably distinguish immune responses to SARS-CoV-2 and other coronaviruses. They are testing patients over a period of several days to follow disease progress and considering lots of questions. Does the degree of the immune response correlate with the severity of the disease? What other biomarkers correlate? Are there early warning signs that indicate which patients are most at risk for bad outcomes? Why does the drug Remdesivir seem to reduce the course of the disease? Does it also reduce the immune response?
With all the data the team is collecting, from microbial sequencing to patients’ personal genomes, they hope to develop much more knowledge about COVID-19 and why certain patients seem to get much sicker, in a very short period of time. The multiplex tests they are developing—assessing many types of cytokines and other markers at once—will also be extremely useful in addressing other diseases.
Cytokine storms are a complication not only of COVID-19 but of respiratory diseases caused by other coronaviruses, such as SARS and MERS. In fact, an out-of-control cytokine response was linked to the high fatality rate for the 2005 outbreak of the H5N1 “bird flu” virus. They are also associated with non-infectious diseases, such as multiple sclerosis and pancreatitis, and are a common side effect of the cancer immunotherapy approach utilizing CAR-T cells. So what we learn about these mechanisms can help advance medical science on several fronts.
Peter Wang is a second-year undergraduate student in The College.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office.
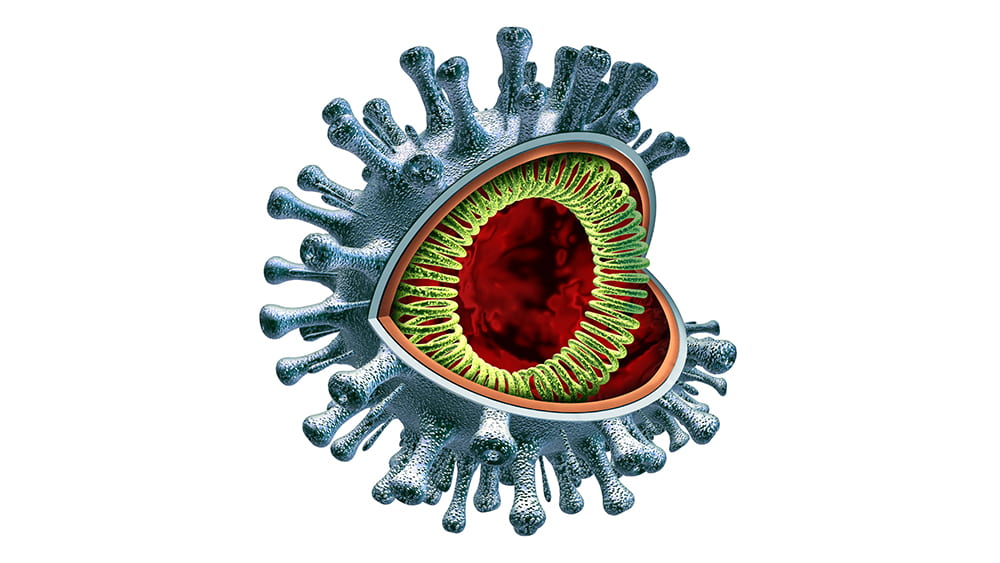
May 13, 2020 | Immunology, Vaccination
by Roma Shah
In this COVID-19 pandemic, the need for virus research has never been greater. People are dying, and right now there seems no effective way to stop it. In New York City, the situation has been overwhelming. Experts suggest this tragedy isn’t over; even if this first wave improves, the coronavirus may reappear, and by then we must understand its mechanisms, its manner of infection, and somehow create a solution.
Michaela Gack, PhD specializes in virology and immunology research, focusing on interactions between viruses and the immune systems of the hosts they infect. She breaks down this pandemic into three component areas: what it is, how people get it, and some ways researchers are looking to intervene.
COVID-19 is caused by a virus that belongs to a larger family of coronaviruses, including those that caused severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), which emerged in 2003 and 2012 respectively. But the COVID-19 virus is a completely new version, and virologists have much to learn to stop this pandemic.
How do people get the disease? The virus, also called SARS-CoV-2, has proven to be highly transmissible, spread easily through the community, and we are just beginning to learn how. Complicating this problem is that a number of people who don’t show symptoms of infection apparently seem able to unknowingly spread the disease.
Although the virus—a very large piece of RNA encased in a protein capsule topped with a crown, or “corona” of spikes— is not “alive” by itself, it is extremely effective in using live cells for energy and spare parts to create many new versions of itself. By working to understand how it operates, Gack and virologists around the country hope to find ways—perhaps multiple ways—to intervene. They are considering several main opportunities.
The first is when the virus first hooks onto a human cell. It does so by attaching to an enzyme called ACE2 which is on the outer surfaces of the cells in the lungs and other organs. Imagine a locked door, where the virus must have the exact key to get into the cell. If scientists can find a way to jam that lock so the key can’t fit, then the cell will stay safely free of the virus.
One approach is “spike-binding”–creating an antibody that will clamp onto the virus’ “keys” and keep them from inserting into the lock. This is how a vaccine works, priming the immune system to make substances that lock onto the keys and keep them from spiking into the cell. But until scientists can develop that vaccine, we can gum up the keys by providing antibodies from patients who have successfully fought off the disease—what scientists call “passive immunity,” until we generate the vaccines to that will help patients to make their own.
Once the virus gains entry into the cell, it must next dissolve its protein coating and release its RNA payload. This is the point where some have suggested hydroxychloroquine might work. The drug, used to treat malaria and lupus, decreases the acidity inside cellular vesicles, working somewhat like a knot in a scarf, to keep the viral coating intact.
Once the RNA is released, it starts replicating uncontrollably, in an interesting way: it uses the building blocks inside the cell to generate long strands of RNA. An enzyme encoded by the virus called polymerase facilitates this process, adding those building blocks along until the chain is complete. Some of these RNA molecules are used to make constituent viral proteins, up to a million of them in each cell.
This is where the drug remdesivir has been hypothesized to work. Remdesivir very closely resembles one of the four main building blocks of RNA. So, when the polymerase is building the chain and looking for the RNA components, it could accidentally grab remdesivir instead, and the replication process falls apart. Think of using screws to put furniture together, where two look very similar. Pick the “wrong” one and the whole desk falls apart.
One more avenue for intervention is in editing the RNA molecules of the virus once they are in the cells. Cellular RNAs contain more than a hundred fifty important modifications at thousands of sites, some with critical regulatory roles analogous to those of protein and DNA modifications. UChicago researchers Chuan He and Tao Pan are world leaders in RNA modifications, and they are now working on ways to deactivate the RNA production that takes over the cellular machinery.
This COVID-19 pandemic, all its unknowns, and all these possible avenues for treatment remind us once again of the important role researchers play even in the most emergent situations, searching for results that can benefit our society as soon as possible.
Roma Shah is a second-year undergraduate studying neuroscience and public policy.









