Research
Interfaces and boundaries between materials, phases, and structural motifs are ubiquitous in our natural and manufactured world. Changes in the atomic and electronic structure at interfaces lead to unique properties such as modified excited state lifetimes, charge transport mechanisms, and interfacial electronic states. In materials, boundaries and interfaces can create unique electronic states that modify charge transfer or facilitate charge recombination. In biological systems, domains and heterogeneity are ubiquitous and critical to function of living systems. In my research group, we aim to investigate how the nanoscale and mesoscale structure of organic, inorganic, and biological materials influences electronic structure, excited state dynamics, chemical reactivity, and functionality.
Domain Imaging in 2D Materials

Polarization-dependent PEEM image of β’- Indium(III) selenide showing antiferroelectric domains on the nanoscale. (Read more here.)
Two dimensional materials hold phenomenal promise in upending the scaling problem in electronic devices and offer unique physics and chemistry critical for next generation energy and information conversion and transport. Realizing the full potential of 2D materials in electronic devices, quantum information, and chemical catalysis, however, is currently roadblocked by a time-intensive and expensive reproducibility problem. This is down to the twin challenges of 2D material synthesis and strong sensitivity of 2D material electronic structure to nanoscale variations in morphology/dielectric environment. In the King Lab, we seek to completely map the connection between nanoscale morphology and 2D materials functionality and offset the reproducibility problem. We use polarization-dependent and time-resolved photoemission electron microscopy to directly image domain formation on the nanoscale and connect nanoscale structure with excited state dynamics and function.
Spectroscopic probes of electrochemical and chiral interfaces
The properties and dynamics of interfaces are at the heart of material and energy transformation, ranging from energy transfer between a battery electrode and a solid or liquid electrolyte to the surface chemistry that can occur at a solid-liquid interface. It is well known that the surface properties of materials can be distinct from those of a bulk material. At material interfaces unique surface electronic states can exist, band-bending can alter the energy of conduction and valence bands relative to the Fermi level, and even a material’s crystal structure can be significantly modified.
These interfacial electronic states are often the “gatekeepers” for charge transfer and reactivity at buried interfaces but selectively investigating their electronic structure and ultrafast dynamics has proved difficult due to a dearth of experimental methods to probe them selectively. The group is developing linear and non-linear techniques that will probe the dynamics at interfaces and uses them to investigate electrochemical and chiral interfaces.
Imaging plasmonic properties of 2D materials
Layered transition metal carbides and nitrides, colloquially called MXenes, are promising new 2D materials for catalysis, particularly for activation of N2 and CO2. Trial and error efforts to optimize MXene catalytic efficiency and selectivity, however, have failed to resolve the simultaneous contribution from both intrinsic material and defects to functionality for the rational design of MXenes for catalysis. In the King Lab, we aim to use nanoscale imaging of MXene electronic structure, dynamics, morphology, N2 and CO2 binding location, and N2 and CO2 activation dynamics to identify the roles of intrinsic material versus defects in N2 and CO2 activation. We will then modify the contributions from intrinsic versus defect sites to N2 and CO2 activation by enhancing catalysis in nanoscale regions with intentional plasmonic fields.
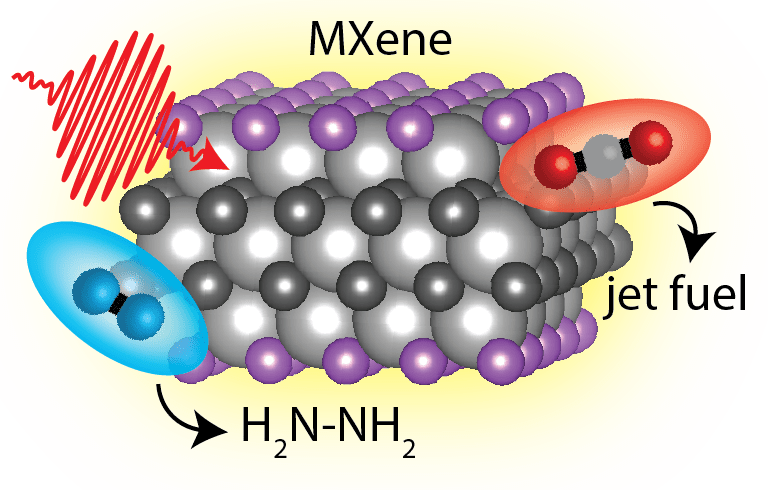
Using TR-PEEM we aim to image the nanoscale electronic structure of MXenes and the mechanisms of CO2 and N2 bond activation.
High-throughput ultrastructural imaging of biological tissue

Photoemission electron microscopy can image ultrathin slices of biological tissue with spatial resolution sufficient to image synapses in neurological tissue. We are working on methods for improving image quality, introducing new contrast mechanisms, and increasing image throughput. The goal is to enable PEEM for volume-electron microscopy and uncover the physical basis for circuits in the brain. This work is in collaboration Bobby Kasthuri in the Neurobiology Department at the University of Chicago.
Funding
We are grateful for funding from the following sources: