The purpose of this literature review is to explain the functions of the rhesus factor and its negative effects on people. It will focus on pregnancies and HDFN, which is a disease caused by the Rh factor. Different treatments for HDFN and trials will be discussed and analyzed throughout the paper.
Function of rhesus factor
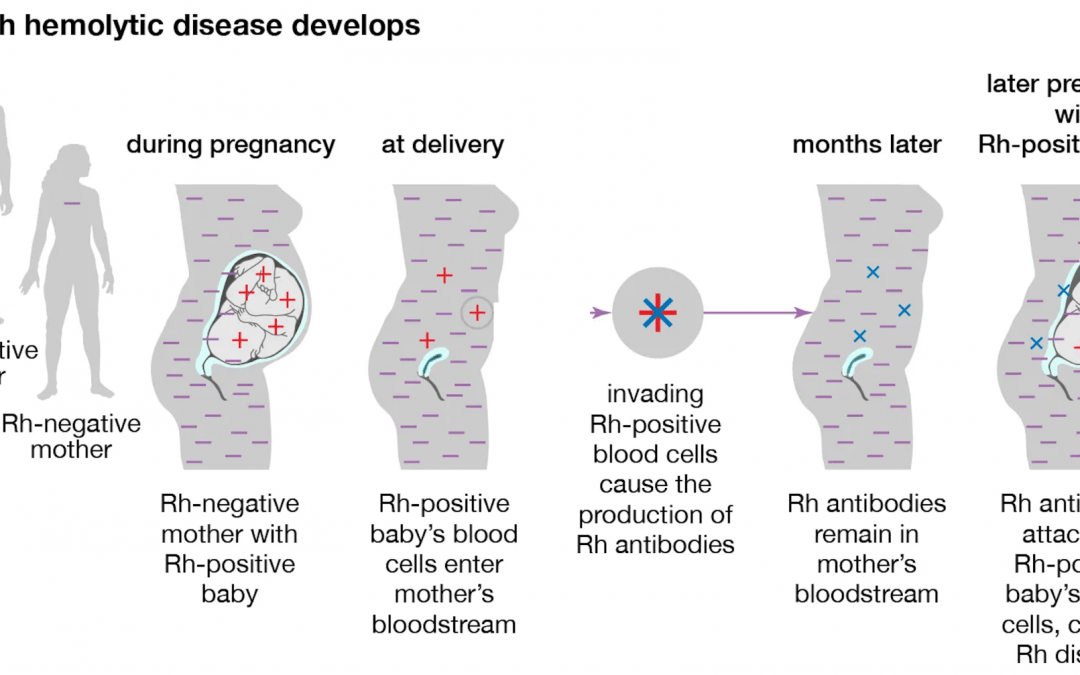
The rhesus factor (Rh factor) is a protein that can show up on a person’s red blood cells. The proteins determine whether the blood of two different people are compatible. This has been the main function of the Rh factor up until recently, when the role of a certain Rh antigen, RhAG, was discovered. RhAG transports ammonium ions through the cell membrane. This function was tested once scientists realized the similarities between RhAg and the amino acid sequence of NH4+ (Karow et al., 2000). That being said, depending on the type of Rh blood group, the functions may differ. Most of the blood groups, however, help identify people who have compatible blood. The RhD factor is the most immunogenic antigen, meaning it is more likely to trigger an immune response than the other antigens. For example, a pregnant woman who is Rh negative, meaning she does not contain the Rh factors on her red blood cells, is not compatible with her fetus, if the fetus is Rh positive, meaning the red blood cells do contain the Rh factor. (Since the Rh factor is an inherited trait, if the mother is Rh negative, the father would have to be Rh positive in order for the fetus to be Rh positive.)
History of rhesus factor
In 1940, the discovery of the rhesus (Rh) blood group system was made by Karl Landsteiner and A.S. Weiner (Britannica et al., 2020). RhD, Rhc, RhC, and RhE are the most common antigens of the 50 that have been discovered. 13 years after the original discovery of the blood group system, of the five most common types of Rh antigens, the most immunogenic one is the Rh D antigen, in 1953, scientists determined that hemorrhages that exposed mothers to the fetus’ red blood cells (given the fetus is is Rh positive and the mother is Rh negative) resulted in the pathogenesis of the rhesus isoimmunization (Dubey et al., 2019).
Once the Rh antigen, specifically the RhD antigen, was seen as harmful in pregnancies, causing HDFN, in 1966, an IgG prophylaxis was invented to prevent sensitization in Rh negative women that was to be used shortly after the delivery of the first child. Since the prophylaxis was administered, there have been several tests and experiments that result in a variety of times, doses, and other recommendations for the drug (Dubey et al., 2019).
Causes and explanation of HDFN
This incompatibility can cause serious health effects for the fetus, the most common effect being hemolytic disease of the fetus and newborn (HDFN). There are several names for the disease, including Rh incompatibility and hemolytic anemia, but HDFN is the most widely-used term for the disease. There is another, similar disease to HDFN, which is called ABO incompatibility. Unlike HDFN, ABO incompatibility often produces little to no symptoms. It is more common than HDFN and only temporary. ABO incompatibility occurs when the mother has O type blood and the fetus has A or B type blood, causing the mother to make antibodies that attack the fetus’ A or B blood cells. HDFN occurs usually after the first pregnancy, which is when the mother is sensitized to the fetus’ Rh positive blood some time during the pregnancy. Then, during the second pregnancy, the mother will create antibodies that can cross through the placenta and attack the fetus’ Rh positive red blood cells faster than the fetus can produce the blood cells. This is why the disease is an anemia. Other effects of HDFN are heart failure, hemorrhaging, premature birth, and or a miscarraige (Sarwar et al., 2021).
Epidemiology of HDFN
A recent 2016 study showed 0.3-0.6% of pregnancies are affected by HDFN (Dubey et al., 2019). About 15% of North Americans and Europeans are Rh negative, compared to 4-8% of Rh negative Africans and 0.1-0.3% of Rh negative Asians (Sarwar et al., 2021). In the US, the frequency of HDFN is more common due to the diversity and the amount of immigration that occurs.
Sensitization in females
A study was done in 2013 to analyze the sensitization of females to determine the timing of RhD immunization during pregnancy and to determine when to administer anti-D prophylaxis. In 51% of the 290 Rhd immunized women experienced sensitization (were exposed to their baby’s Rh positive blood) during the first pregnancy, while 33% of the women experienced sensitization during the second pregnancy. In 94% percent of the pregnancies, Rh antibodies were developed after the first trimester. 73% of the women developed antibodies in the second or third trimester, while 21% developed them during or after delivery. The data collected gives reason for doctors to administer anti-D prophylaxis in the beginning of the third trimester (28-30 weeks) to all Rhd positive women carrying RhD negative fetuses (Tilbad et al., 2013).
Current treatments for HDFN
Anti-D prophylaxis is an IgG prophylaxis that works to prevent the sensitization that occurs during the first pregnancy of a Rh negative mother with its Rh positive fetus. It is a two-does treatment that is administered during the third trimester of the pregnancy: one injection of the immunoglobulin is given on the 28th week of pregnancy, and the second is administered about six weeks after the first. The drug is meant to prevent the sensitization of the antigens, so if the mother is already sensitized, then the drug is virtually useless. Because of this, several trials have been run to determine the ideal time in which the prophylaxis should be administered.
A 2014 study tested the effectiveness of postnatal RhD prophylaxis, meaning the drug is administered after the first pregnancy, but before the second. The reason for this timing is because the prophylaxis targets the antibodies that are formed after the sensitization that occurs during the first pregnancy. 89 pregnancies were monitored for the trial. Of the 89, 56 of pregnancies (63%) were sensitized during the first pregnancy, 21 (24%) were sensitized during the second pregnancy, and 12 (13%) were sensitized during later pregnancies. Rh incompatibility occurred in 28 of the pregnancies (31%), and 25 of those cases were a direct cause of the sensitization in the previous pregnancy (Dajak et al., 2014).
Another treatment that has been tested for both HDFN and ABO incompatibility is exchange transfusion. Exchange transfusion completely replaces 100% of the blood circulating in a body with blood that is compatible with whatever the blood may be interacting with. Exchange transfusion cannot be performed on fetuses, so the procedure is done after birth
In 2007, exchange transfusion was used on 25 cases. The table below shows the results of the 25 cases that were given an exchange transfusion (Sharma et al., 2007).
20 (80%) of the cases needed only one transfusion, and the other 5 (20%) needed a second transfusion. 15 of the cases were cases of HDFN. Of the 15, 11 (73%) needed one transfusion, while the other four (27%) needed a second. One of the cases was suffering from neonatal septicemia in addition to HDFN. The newborn died due to “septicemia and respiratory stress”.
Discussion and future treatments
HDFN can be a fatal disease for fetuses, and unfortunately there is no way to prevent the Rh factor from causing HDFN because it is hereditary. The only way to prevent HDFN is to prevent sensitization. The anti-D prophylaxis is a successful treatment as the trials have proven, however, it is more of a preventative measure. It is also not available globally. In the US, the prophylaxis is attainable for the majority of Americans, but everywhere else, the prophylaxis is uncommon and quite rare. Developing countries are having trouble gaining access to this specific treatment. Knowledge about treatments like this is necessary, so it needs to be shared and more widely produced in other countries.
For future research, a treatment should be developed for fetuses and newborns that have already been diagnosed with HDFN. The exchange transfusion is somewhat successful, but it still has yet to be improved, which is crucial because of how big the transfusion is.
Annotated Bibliography
Britannica, The Editors of Encyclopaedia. “Rh blood group system”. Encyclopedia Britannica, 9 Apr. 2020
Dajak, Slavica et al. “The importance of antenatal prevention of RhD immunisation in the first pregnancy.” Blood transfusion =Trasfusione del sangue vol. 12,3 (2014): 410-5.
Dubey, . “Haemolytic Disease of the Fetus and Newborn: Past, Present and Future Considerations”. Acta Scientific Medical Sciences 3.10 (2019): 153-161.
Flegel, Willy A. “The genetics of the Rhesus blood group system.” Blood transfusion = Trasfusione del sangue vol. 5,2 (2007)
Karow, Julia. “A Role for the Rhesus Factor”. Scientific American, 31 Oct. 2000
Sarwar A, Citla Sridhar D. Rh-Hemolytic Disease. [Updated 2021 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022
Sarwar, Ayesha. and Divyaswathi Citla Sridhar. “Rh-Hemolytic Disease.” StatPearls, StatPearls Publishing, 14 August 2021.
Sbarsi, Ilaria et al. “Implementing non-invasive RHD genotyping on cell-free foetal DNA from maternal plasma: the Pavia experience.” Blood transfusion = Trasfusione del sangue vol. 10,1 (2012)
Sharma, D C et al. “Study of 25 cases of exchange transfusion by reconstituted blood in hemolytic disease of newborn.” Asian journal of transfusion science vol. 1,2 (2007)
Tiblad, Eleonor et al. “Consequences of being Rhesus D immunized during pregnancy and how to optimize new prevention strategies.” Acta obstetricia et gynecologica Scandinavica vol. 92,9 (2013)
Turner, Rebecca M et al. “Routine antenatal anti-D prophylaxis in women who are Rh(D) negative: meta-analyses adjusted for differences in study design and quality.” PloS one vol. 7,2 (2012)
Image: https://www.britannica.com/science/Rh-blood-group-system/images-videos#/media/1/500915/95401