
Jul 2, 2020 | COVID-19, Vaccination
By Elise Wachspress with Tinyan Dada
If you haven’t been infected yet, congratulations, you’ve made it this far.
We’ve streamlined our grocery store visits, maintained a six-foot distance at all times, even bought or made patterned, reusable masks for the family. We have a COVID routine, but we’re still waiting for the magic switch that will make everything normal again.
Thousands of scientists and technicians around the world are working to develop vaccines—substances that train our immune cells to attack particular pathogens—to keep us from getting COVID-19. Scientists continue to find multiple strains of the SARS-CoV2 virus circulating in various populations; it is becoming clear we may need multiple vaccines. And how long will these protect us? Measles inoculation works pretty much forever, but that is a rarity.
Sadly, even the most effective vaccination programs won’t work for everyone: the very young, the old, and especially those with impaired immune systems, for whom they may even cause disease. Will these people have to live forever in a COVID-19 bubble?
Maybe not. If we can establish herd immunity—where enough of the population is resistant to the disease—all of us might be able to resume a normal life.
But how many is “enough”? In an article in Immunity last month, UChicago geneticist Luis B Barreiro, PhD and grad student Haley E Randolph lay out the many parameters affecting herd immunity. In the case of this novel coronavirus, potentially influenced by countless unknowns—social structure, population density and age, even the genetic vulnerabilities of particular ethnic groups—assessing the potential for herd immunity becomes particularly uncertain and complicated.
The starting point, says Barreiro and Randolph, is to identify the average number of new cases each infected person might cause. In a completely susceptible population, where no one has experienced the virus before, this average is designated as R0. But once people start becoming resistant—either by getting the disease or through a vaccine—the effective reproduction rate, Re, starts to go down. The goal of vaccination programs is to get Re down below one, meaning more people have the disease than are transmitting it, and the disease curve begins to arc toward zero.
In the case of COVID-19, R0 has so far been estimated across different populations to be anywhere from around two to nearly six. (All of these R0 estimates were generated of course, without anyone understanding whether or how many people without symptoms can transmit the disease.)
So, Barreiro and Randolph suggest, suppose we take an average of the average—this totally new pathogen is forcing everyone to make a lot of guesses—and settle on an R0 of three. Mathematically, that would mean that incidence of the infection would begin to decline when about 67 percent of the population was resistant, and we would have a start—no promises!—toward herd immunity.
Complicating these models further are super-spreader events, like when one person singing in a church choir inadvertently infected at least 52 people with the coronavirus. We know from experience with MERS or even measles that one person or a tiny group can sometimes drive an inordinate number of infections. We don’t yet know how common these events are with COVID-19, although we have now learned that forcefully expelled droplets are a major mode of transmission, so taking precautions like wearing a mask, singing only at home, or even speaking more softly can ostensibly reduce R0.
Another important unknown is how long antibodies to the coronavirus might last. A year? Two? Because this coronavirus is so new, we have little idea how long the resistance we build up—either from having the disease or getting a vaccine—might last. Either way, protecting the vulnerable will depend on maintaining herd immunity over time.
Barreiro and Randolph go on to explain how to assess the infection fatality rate—the proportion of infected people who die of the disease—a metric critical in assessing the cost to society. Without isolation strategies, they project that worldwide deaths could exceed 30 million. Of course, as was the case in Italy, timing is everything: the faster the infection rate builds, the less likely the health care system can care for all the infected, and the more people will die.
In summary, Barreiro and Randolph point out that herd immunity is likely to work in only concert with a viable vaccination strategy, spread broadly throughout the population. Sweden’s coronavirus strategy involved keeping restaurants and businesses open, hoping that if less vulnerable members of the population interacted out in the community, they would generate some degree of herd immunity. So far, only around six percent of Swedes have developed COVID-19 antibodies, leaving the rest of the population at risk for serious illness and death and Swedes persona non grata visitors to other European countries.
So, in the short term, we continue to embrace our COVID routine. It is not much fun, but it may save our lives and the lives of others.
Tinyan Dada is a second-year undergraduate student at UChicago.
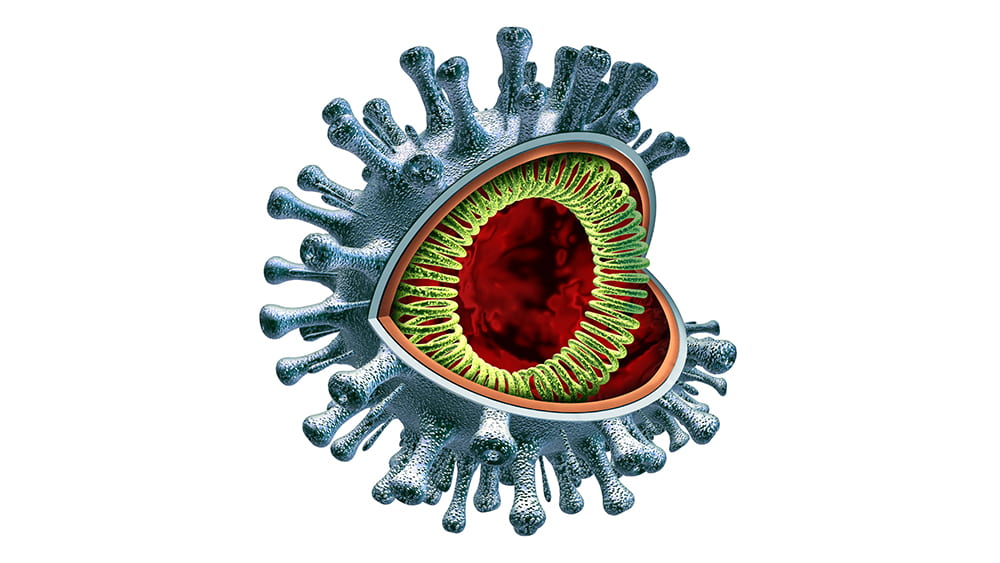
May 13, 2020 | Immunology, Vaccination
by Roma Shah
In this COVID-19 pandemic, the need for virus research has never been greater. People are dying, and right now there seems no effective way to stop it. In New York City, the situation has been overwhelming. Experts suggest this tragedy isn’t over; even if this first wave improves, the coronavirus may reappear, and by then we must understand its mechanisms, its manner of infection, and somehow create a solution.
Michaela Gack, PhD specializes in virology and immunology research, focusing on interactions between viruses and the immune systems of the hosts they infect. She breaks down this pandemic into three component areas: what it is, how people get it, and some ways researchers are looking to intervene.
COVID-19 is caused by a virus that belongs to a larger family of coronaviruses, including those that caused severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), which emerged in 2003 and 2012 respectively. But the COVID-19 virus is a completely new version, and virologists have much to learn to stop this pandemic.
How do people get the disease? The virus, also called SARS-CoV-2, has proven to be highly transmissible, spread easily through the community, and we are just beginning to learn how. Complicating this problem is that a number of people who don’t show symptoms of infection apparently seem able to unknowingly spread the disease.
Although the virus—a very large piece of RNA encased in a protein capsule topped with a crown, or “corona” of spikes— is not “alive” by itself, it is extremely effective in using live cells for energy and spare parts to create many new versions of itself. By working to understand how it operates, Gack and virologists around the country hope to find ways—perhaps multiple ways—to intervene. They are considering several main opportunities.
The first is when the virus first hooks onto a human cell. It does so by attaching to an enzyme called ACE2 which is on the outer surfaces of the cells in the lungs and other organs. Imagine a locked door, where the virus must have the exact key to get into the cell. If scientists can find a way to jam that lock so the key can’t fit, then the cell will stay safely free of the virus.
One approach is “spike-binding”–creating an antibody that will clamp onto the virus’ “keys” and keep them from inserting into the lock. This is how a vaccine works, priming the immune system to make substances that lock onto the keys and keep them from spiking into the cell. But until scientists can develop that vaccine, we can gum up the keys by providing antibodies from patients who have successfully fought off the disease—what scientists call “passive immunity,” until we generate the vaccines to that will help patients to make their own.
Once the virus gains entry into the cell, it must next dissolve its protein coating and release its RNA payload. This is the point where some have suggested hydroxychloroquine might work. The drug, used to treat malaria and lupus, decreases the acidity inside cellular vesicles, working somewhat like a knot in a scarf, to keep the viral coating intact.
Once the RNA is released, it starts replicating uncontrollably, in an interesting way: it uses the building blocks inside the cell to generate long strands of RNA. An enzyme encoded by the virus called polymerase facilitates this process, adding those building blocks along until the chain is complete. Some of these RNA molecules are used to make constituent viral proteins, up to a million of them in each cell.
This is where the drug remdesivir has been hypothesized to work. Remdesivir very closely resembles one of the four main building blocks of RNA. So, when the polymerase is building the chain and looking for the RNA components, it could accidentally grab remdesivir instead, and the replication process falls apart. Think of using screws to put furniture together, where two look very similar. Pick the “wrong” one and the whole desk falls apart.
One more avenue for intervention is in editing the RNA molecules of the virus once they are in the cells. Cellular RNAs contain more than a hundred fifty important modifications at thousands of sites, some with critical regulatory roles analogous to those of protein and DNA modifications. UChicago researchers Chuan He and Tao Pan are world leaders in RNA modifications, and they are now working on ways to deactivate the RNA production that takes over the cellular machinery.
This COVID-19 pandemic, all its unknowns, and all these possible avenues for treatment remind us once again of the important role researchers play even in the most emergent situations, searching for results that can benefit our society as soon as possible.
Roma Shah is a second-year undergraduate studying neuroscience and public policy.

Apr 30, 2018 | Genetics, Medicine, Microbiome, Vaccination
A selection of health news from the University of Chicago and around the globe curated just for you.
Doctors try to lower $148K cancer drug cost; makers triple price of pill
A group of cancer doctors, including Mark Ratain, found a blood cancer drug called Imbruvica, which typically costs $148,000 a year, could be just as effective at a lower dose. Drug makers found out and introduced a new pricing scheme that ensures dose reductions won’t save patients money or impact company revenue. (The Washington Post)
UChicago startup raises $750K to treat migraines with a nasal spray
Seurat Therapeutics, which will compete in the Polsky Center’s New Venture Challenge this spring, announced Wednesday that it has raised its first round of funding that will allow the company to begin testing their product in human clinical trials. (ChicagoInno)
Specific bacteria in the small intestine are crucial for fat absorption
New research by Eugene Chang and colleagues shows how the typical calorie-dense western diet can induce expansion of gut microbes that promote the digestion and absorption of high-fat foods. (The Forefront)
Genetic screening tool identifies how the flu infiltrates cells
Researchers at UChicago have developed a genetic screening tool—using CRISPR/Cas9—that identified two key factors that allow influenza virus to infect human lung cells. (The Forefront)
Weight might not be why obesity damages knees
The gut microbiome could be the culprit behind arthritis and joint pain that plagues people who are obese, according to a new study from the University of Rochester Medical Center. (Futurity)
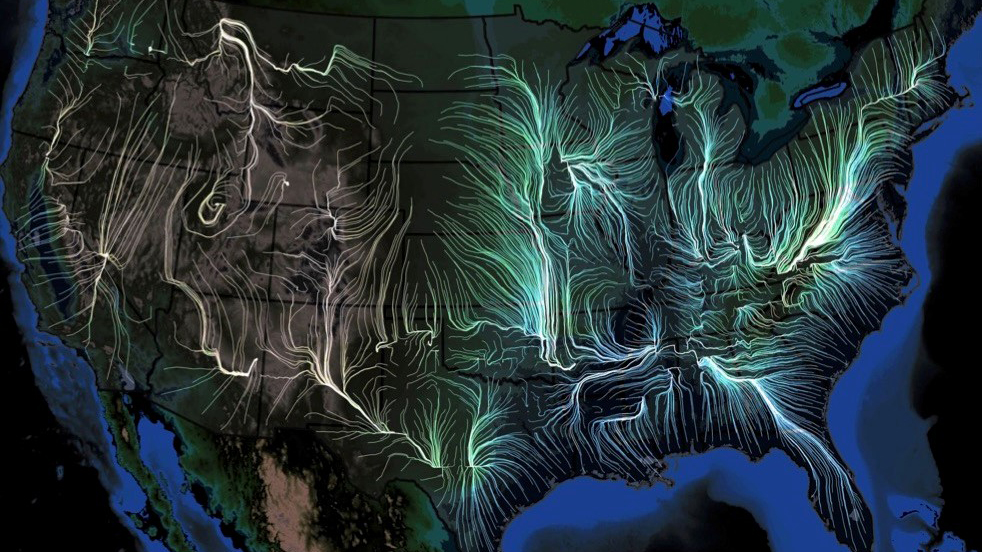
Mar 9, 2018 | Big Data, Vaccination
by Elise Wachspress
Last week, the scientific journal eLife (established by the Howard Hughes Medical Institute, Max Planck Society, and the Wellcome Trust) published a remarkable study—a veritable “weather forecasting” tool to predict, in detail, the movement of the flu in the U.S.
Scientists from the University of Chicago, Microsoft Research, Columbia University, and Argonne National Laboratory used many gigantic, disparate datasets—insurance records of more than 150 million people, 1.7 billion geo-located Twitter posts, air traffic, weather, how often people visit friends nearby, vaccination rates, even mutations in the virus itself—over nine flu seasons, from 2003 to 2011, to create a model to describe in detail how flu epidemics start and spread.
The team then verified the accuracy of their computational model by “predicting” the spread of flu for three subsequent seasons (2014-2017), predictions which dovetailed with what actually happened, at resolutions higher than those created by the Centers for Disease Control and Prevention.
The scientists’ work documented that seasonal flu outbreaks generally originate in warm, humid areas of the south and southeast, mostly near the Gulf of Mexico or the Atlantic Ocean; though infections can originate in many places, they usually gain traction and grow into epidemics only near large bodies of water. Of the factors most important in spreading the virus beyond these coastal areas, foremost was the original host population’s “social connectedness”—how often people interacted on a day-to-day basis. The next most critical factors were weather (high humidity, high temperature, and low barometric pressure fostered the epidemic); changes in the virus itself; and land travel by the host population, which turned out to be much more powerful in fueling an epidemic than airplane travel.
The new model they derived offers a great tool to help public health officials improve prevention efforts, suggesting, perhaps, how best to deploy flu shots early on, and how people near the coasts should think about their local travel and socialization once people start getting sick. In fact, the model could be used to test prospective public health policies in silico before they are implemented, saving precious energy and resources.
But perhaps as interesting as the forecasting tool is the scientific “network” that created it.
The strength of weak ties
When you are looking for a job, identifying potential business investors, even finding a play group for your kids, it’s unlikely your spouse, siblings, or even immediate friends can help much. They know many of the same people and ideas you do. We’ve learned the power of networking, reaching out to those somewhat distant from us, for better answers. The internet and social media like Pinterest and YouTube have brought networking to a whole new level: they provide easy access to all kinds of far-flung, elusive information, from how to help your kids with their math homework or make crepe suzettes, to how to do your taxes or evaluate your investment portfolio.
Networks work most effectively when they include lots of weak, distant ties. The more people from disparate backgrounds you can reach even briefly or intermittently, the more likely you are to gain more knowledge and better ideas.
Andrey Rzhetsky, PhD, the leader of the flu model team, has mastered the art and science of networking.
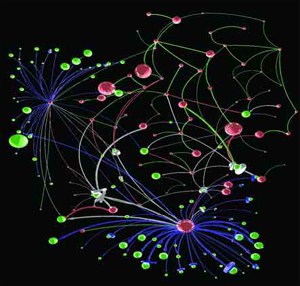
Rzhetsky’s network map of patterns of gene expression in brain tissue of people with autism, from a 2010 paper
He began his career as a mathematical biologist, analyzing gene sequences and connecting the dots, when computational biology was still in its infancy. By 1991 he was harnessing microcomputer programs to construct “phylogenetic trees”—categorizing differences among organisms—to better understand evolution. As more and more scientific research was published online, Rzhetsky became among the earliest developers of text mining, an automated way to search journal articles, capturing and cataloguing connections between genes, proteins, and diseases, without a human having to read each article.
He began training machines to suss out connections between all kinds of data. The “networks” of information he built ranged from molecular interactions in biological systems, to documenting how seemingly unrelated diseases overlap, to the reliability of scientific studies and much more, informing a growing variety of fields. What environmental factors might trigger autism or bipolar disorder? Which diseases inflict the greatest human suffering? How should scientists choose experiments to best accelerate the progress of science and medicine? Rzhetsky and his collaborators have mined and harnessed data to find useful answers to these disparate questions and more.
And the number and diversity of his collaborators on these projects is pretty stunning. Rzhetsky is ingenious at using weak ties to find expertise all over the world. Whether it is getting access to huge caches of medical records that stretch over generations (Denmark) or computational/artificial intelligence experts at major corporations (Seattle), Rzhetsky uses the power of weak ties to build bases of knowledge that push the boundaries of science. His cohort on the flu research provides an example:
- Rzhetsky’s closest partner was Ishanu Chattopadhyay, PhD, now also a faculty member at UChicago. Chattopadhyay has developed algorithms for a broad swath of areas: path-planning for mobile robots, autonomous decision-making, swarming behavior and self-organization, and reverse-engineering measurements of populations, species, and gene expression to understand systems’ mechanisms.
- Josh Elliott, PhD, started his career as a high-energy theoretical particle physicist. Over the past decade he’s applied those analytical skills to climate change, agricultural production, economic modeling, and the social sciences as a research scientist in the University of Chicago’s Energy Policy Institute.
- Emre Kiciman, PhD, a principal researcher in artificial intelligence at Microsoft Research, lent his expertise in social media analysis, particularly attuned to its inherent systematic biases.
- And Jeffrey Shaman, PhD, of Columbia University, provided proficiency in climate, atmospheric science, hydrology, and other environmental determinants of infectious disease transmission and forecast.
Scientific successes like theirs demonstrate that novel breakthroughs depend more and more on teams with diverse expertise, experiences, and viewpoints. Just as casting a wide net leads us to new recipes (and ways to avoid the flu!), so those on the forefront of discovery must reach across departments, institutions, even national boundaries to achieve the greatest knowledge.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office
Feb 21, 2018 | Medicine, Vaccination
by Elise Wachspress
Staphylococcus aureus (commonly called Staph) is found on the skin and in the nose, throat, and gastrointestinal tract of about a third of the human population. Staph is one of the many “commensal” bacteria that routinely make their homes in our bodies, usually without incident.
But if Staph grows unchecked, it can cause problems. Staph infections on skin and other soft tissues account for about 14 million outpatient or emergency visits in the U.S. each year, and some populations, especially those in military training, experience significantly greater risks.
Staph can also become more invasive, infecting deep tissues, the lungs, blood stream, skeletal structures and joints, urinary tract, or heart lining. It can even cause sepsis, an especially dangerous systemic condition. Low-birth-weight babies, nursing homes residents, surgical patients, diabetics, cancer patients, or anyone with “foreign” appliances in their bodies (like implants, catheters, or ventilators) are more vulnerable to these kinds of Staph infections.
Generally, our bodies are quite adept at building weaponry—antibodies—against bacteria and viruses. Once the body produces these defenders, they tend to become part of a lifelong arsenal, effective deterrents against future infection by that specific interloper. But although healthy people from infant to adults carry Staph antibodies, these often fail to keep this particular bacteria from re-establishing itself. In fact, once you’ve had a Staph infection, you are more likely to get the infection again, even if you’ve been effectively treated with surgery or antibiotics.
The rise of antibiotic-resistant strains—namely methicillin-resistant Staph aureus, commonly known as MRSA—makes this problem especially worrisome. We are running out of antibacterial armaments to stop MRSA infections, and the cost of the fight is astronomical, estimated at over $10 billion per year in the U.S. alone. Finding a new way to reduce MRSA infections is critical.
University of Chicago microbiologists Olaf Schneewind, MD, PhD, and Dominique Missiakas, PhD, have found that a protein, SpA, which rides on the surface of the Staph bacterium blocks the development of effective antibodies. The Schneewind/Missiakas team cleverly generated mutant proteins, with new chemical groups at the binding sites of SpA, disordering the bacterium’s ability to block the development of antibodies. Early studies show these new proteins are effective in helping the immune system respond much more effectively to Staph colonization, and now the team is tweaking the molecules to find the most powerful version.
With the support of the Polsky Center for Entrepreneurship and Innovation, they have now launched a new company, ImmunArtes, to move their discovery toward commercialization. In December, the team won a $175,000 investment from the University of Chicago Innovation Fund.
In theory, the modified molecule could be a twofer: the basis for a vaccine against MRSA and a potential treatment for those already infected. The team recognizes the problem implicit in the latter strategy: MRSA can become lethal so quickly that the patient might die before the body can make enough antibodies.
The vaccine strategy also presents challenges. Several pharmaceutical companies (Merck, GlaxoSmithKline, and Novartis) have tried in vain to develop Staph vaccines or immune therapeutics; others (Pfizer and MedImmune), are still trying. What makes the prospect of a successful vaccine or antibody therapy so daunting is the multitude of evasive mechanisms deployed by Staph. Nevertheless, Schneewind and Missiakas are confident their vaccine strategy can overcome these obstacles, help decolonize Staph in humans, and reduce the risk of new Staph infection. The team is launching an aggressive development plan, producing clinical grade vaccine, generating preclinical data, and launching a human trial within the next three years.
The value of an effective vaccine would be tremendous. The nursing home industry is already taking note, and gyms, schools, and daycare centers will certainly be important beneficiaries of a MRSA vaccine.
Success will put the world one tool closer in the battle against antibiotic-resistant superbugs.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office
Jan 17, 2018 | Vaccination
By Elise Wachspress
Americans often associate parasite infections with poorly resourced communities in underdeveloped countries. But some parasites are not uncommon in the U.S.
Toxoplasma is one which many Americans may not hear about until a prenatal visit—even though over 60 million people in the U.S. are infected. The usually mild indications of infection are generally not recognized as “symptoms,” and for healthy individuals, the parasite usually retreats into self-enclosed cysts in the brain, eye, or muscles.
But for those with weak or underdeveloped immune systems—like those with cancer, HIV, or especially a fetus—Toxoplasma is quite dangerous. In a fetus unwittingly exposed via its apparently healthy mother, the infection can cause death, loss of sight, or severe neurologic deficits.
Vulnerability usually arises when the mother is exposed to Toxoplasma for the first time during her pregnancy, before her body can generate the immune response to sequester the parasite into cysts. Pregnant women are thus advised not to handle or eat raw or undercooked meat, seafood, or milk and to avoid exposure to material potentially contaminated with cat feces, including soil. Of all the many animals that can be infected with Toxoplasma, members of the cat family are the only ones in which the parasite reproduce sexually, and kittens are the most likely to excrete active parasites.

Left: a form of toxoplasmosis-causing parasite that can be transmitted through the placenta to a fetus. Right: Toxoplasma parasites (green) inside a cyst. (Rima McLeod/University of Chicago)
Rima McLeod, MD, medical director of the University of Chicago Medicine Toxoplasmosis Center and a professor of both ophthalmology and pediatrics, has spent a good part of a very active career on these one-celled animals. She and the international teams with whom she works—many of which she leads—have looked at Toxoplasma from a variety of angles: its epidemiology (Are fathers of babies with the infection likely to have it? Yes.), new animal models (Zebra fish, with their short lifespans and transparent bodies, are proving helpful for drug testing), diagnosis, treatment, and very importantly, vaccines.
Diagnosis
In France and Austria, where prenatal screening for Toxoplasma is the law, symptomatic disease has been nearly eliminated. One of McLeod’s research teams recently made a strong economic case for using an inexpensive, point-of-care diagnostic test, developed and approved in France, for all pregnant women, not only in the U.S., but in low- and middle-income countries as well. They found this simple immunological test 100 percent sensitive and specific. The cost factor is especially critical, as the test should be repeated monthly over the course of pregnancy, to identify any potential exposures while the baby is developing—the worst possible time to come in contact with these tiny animals. Babies infected with active Toxoplasma can then be treated effectively.
Unfortunately, Toxoplasma cysts, commonly located in the muscles, eyes, or brains, are usually permanent—so far, nothing can reliably eradicate them. This leaves anyone who carries the cysts—babies or adults—vulnerable to attacks from escaping parasites when immunity is low—think cancer, HIV, malnutrition, treatments for autoimmune disease.
Finding effective drug treatments that work on the cysts is therefore a priority, and McLeod and colleagues have identified a small molecule that inhibits both the parasite’s dormant and active phases—a molecule which also appears to have significant relevance in attacking the malaria parasite. A child dies of malaria every eleven seconds, with up to half a million children lost every year, so synthesizing this molecule into a viable drug could improve or save billions of lives. McLeod is now working with the Polsky Center for Entrepreneurship and Innovation to get their help in developing a safe, effective oral medicine that protects against both diseases.
A Vaccine—or Several—on the Way
McLeod and her many collaborators are advancing several vaccination strategies. One approach is to vaccinate cats so they don’t shed the parasite, which will prevent environmental contamination with Toxoplasma in their hardiest, most infectious stage.
She is also leading an effort aimed at developing a human vaccine against the parasite. The self-assembling protein nanoparticle her team has designed and produced has been proven to work in mice with human genes, although there is still work to be done to assure the vaccine works in people.
A Key to Many Diseases?
Perhaps most tantalizing is McLeod’s “systems biology” research. She has been leading a large team assembled from around the world to create a comprehensive analysis of the toxoplasmosis-infected brain, from the genes that cause disease susceptibility; to the proteins those genes encode; to how these molecules affect the neural, immune, and endocrine networks; to identifying biomarkers of infection and disease.
They have found significant crossover between the biochemical pathways involved in toxoplasmosis and those in many other neurological disorders: proteins associated with the neurodegeneration in Parkinson’s and Alzheimer’s diseases, others involved with epilepsy, and still others involved in cell proliferation and cancer. Thus, deep discovery in the basic science of toxoplasmosis may offer a roadmap to understanding many neuropathologies and give clues to treat or prevent them.
Who knew that a parasite that causes brain cysts could be so useful?
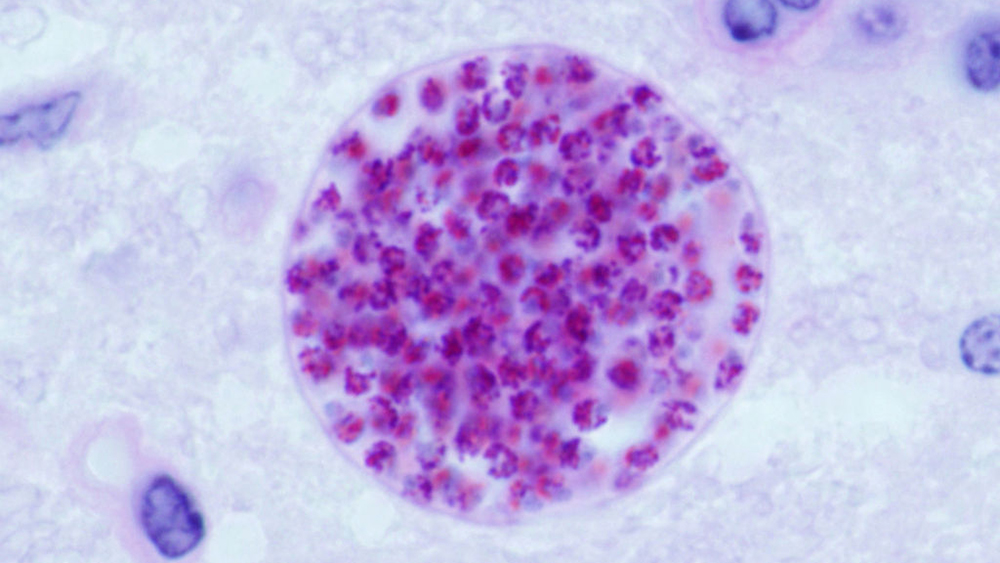
Photo above: Microscopic cysts containing Toxoplasma gondii develop in the tissues of many vertebrates. Here, in mouse brain tissue, thousands of resting parasites (stained red) are enveloped by a thin parasite cyst wall. (Jitinder P. Dubey/Wikimedia Commons)
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office











