Feb 5, 2020 | Microbiome, Neuroscience
by Elise Wachspress
If you read the news—or this blog—then you know that researchers are identifying more and more interactions between the brain and the gastrointestinal tract. Some of these linkages are directly neurological; many others are chemical interactions caused by digestive byproducts of the bacteria in our guts.
A large group of scientists from the University of Chicago (led by Issam Awad, MD), the University of Pennsylvania, and universities and academic medical centers from New York to San Francisco and Australia to Germany have found another mechanism on the path from the gut to the brain—and with it, perhaps a new way to intervene in diseases that affect the most inaccessible organ of our bodies. Their report was the cover story of Science Translational Medicine this past November.
The story starts with decades of research on a rare condition, cerebral cavernous malformations (CCM). CCM causes enlarged, irregular clusters of capillaries, the tiniest blood vessels. CCM capillaries have abnormally thin walls, without the elastic fibers that make the vessels pliable. These irregularities, which make the vessels prone to lesions and blood leakage, can occur throughout the body. But they are by far most worrisome in the brain and spinal cord, where the leaks can lead to seizures, stroke, hearing or vision loss, even paralysis.
CCM is caused by genetic mutations in one of three different genes, but one of these is not like the others. Patients with mutations in the PDCD10 gene usually suffer much earlier, more severe health events, like brain hemorrhage.
A disorder with such disastrous outcomes linked so closely to single gene mutations encouraged researchers to develop mouse models for each of the three mutations. The models could help them discover the molecular mechanisms involved in each—especially what was different in the PDCD10 mice—and exploit what they learned to develop new treatments and preventive measures for CCM.
But examining the brain slices of the mice under the microscope was expensive and time-consuming. An Australian colleague noted that he had heard of instruments that could do x-ray microcomputed tomography on tiny subjects. Awad discovered only one in the entire Midwest, which happened to be in the basement of ivy-covered Culver Hall, one of the oldest buildings on the UChicago campus. Evolutionary paleobiologist Zhe-Xi Luo, PhD had acquired this microCT (now dubbed a Paleo CT!) to examine tiny, delicate insect fossils. A partnership was born, and, with Luo’s support, Awad’s team had a much faster way to assess precisely the amount of bleeding in each mouse brain and the correlation with various genetic, dietary or pharmacologic manipulations.
The investigators found that mice that developed disease did, in fact, share a characteristic microbiome. These bacteria produced a lipopolysaccharide—often called an endotoxin—on their outer membranes, a likely culprit in fostering disease progression. But the fact that the microbial communities were similar among all three genetic models indicated that it wasn’t the microbes alone that made mutant PCDC10 so much more virulent than the other two mutations.
The team moved on to test whether the PDCD10 mutation might cause some kind of rupture in the mucus membranes coating the gut, and there they found the smoking gun. The mutant gene did, in fact, attack the gut’s mucus layer, allowing the offending microbes access to the deeper tissues of the colon. With the integrity of the gastrointestinal tract compromised, bacterial byproducts could leak into the mice’s blood stream and be carried to the brain.
Awad’s team is currently extending this research in mice to patients with CCM. They hope to see how the microbiome can be used as a biomarker of disease and how targeting therapies to the gut might prevent brain bleeding. In the meantime, they think CCM patients might want to avoid polysorbate 80. They might also try any conditions or foods—or drugs—that foster mucus production, which might be helpful in preventing further degradation not only in the gut, but also the capillaries in the brain.
There are implications for the rest of us. The Awad team has found genetic anomalies in the aging brain similar to those in CCM. So what they learn about the mechanisms of this rare disease might also be used to prevent brain bleeding in the rest of us as we age. By carefully manipulating PDCD10 and the mucus layers of the gut, we may one day learn how to better care for what is arguably the most important and delicate organ in the body—the brain.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office

Jan 22, 2020 | Microbiome, Neuroscience
by Helen Robertson
What is your biggest concern about growing old? A decline in physical fitness? A loss of independence? Or perhaps it’s the fear that your mental fitness might start to lose its edge?
For the 50 million people worldwide living with dementia, that last scenario is a reality. A dementia diagnosis comes with big personal, social, and financial consequences: the cost of care for someone living with dementia is reportedly higher than that of both heart disease and cancer combined.
The most common cause of dementia in the US is Alzheimer’s disease. Although its symptoms are well known—cognitive decline, neuroinflammation, and the tell-tale formation of amyloid plaques, the hard aggregation of proteins between nerve cells in the brain—the precise cause remains unknown, and there is no current cure.
As the world population continues to age, dementia is increasing. The need to uncouple its complex biological processes is urgent.
Sangram Sisodia, PhD, has spent the past three decades investigating just that. But in recent years his Alzheimer’s research has taken an exciting and unexpected focus: the gut.
Thanks to recent findings, many of them by research teams at UChicago, we have learned that the bacteria living in our guts can affect many aspects of health. Normally, our gut microbiome contributes to everyday wellbeing and immunity. But just like any other community, the composition of our microbiome can fluctuate on a near-daily basis. And when a shift in balance occurs, things can go awry.
Our intestinal microbes have a particular influence on immunity and neurological function, both important factors in Alzheimer’s. Those with the disease have also been found to experience a change in the character of their gut microbiome.
That’s where Sisodia stepped in.
Over the past few years, his team has been using mouse models of Alzheimer’s to understand how the composition of our gut microbiome might influence neurological inflammation caused by certain immune cells. They thought this inflammation could contribute to both the protein deposition and neurodegeneration in Alzheimer’s.
Sisodia’s research has already generated some interesting findings. His studies, published in Scientific Reports and the Journal of Experimental Medicine, showed that the long-term treatment of mice with broad-spectrum antibiotics reduced neuroinflammation and slowed the growth of amyloid plaques.
After treatment, the mice also showed significant changes in the composition of their gut microbial communities. Some types of bacteria completely disappeared; others multiplied—suggesting bacterial diversity in the gut plays a role in the immune response during disease progression.
But only for male mice. In females, antibiotics actually increased the inflammatory response, with no change in brain plaques. With Alzheimer’s more prevalent in women than men, this gender difference in immune response clearly warrants more study.
Myles Minter, postdoctoral scholar in Sisodia’s lab who is now a research analyst at William Blair, wondered what might happen if one could prevent Alzheimer’s by treating it early—really early. He gave two-week-old mice pups antibiotics for just one week—which left lifelong effects on both their gut microbiome and amyloid plaque formation.
But this is no simple solution. UChicago neonatologist Erika Claud has shown how changes to the microbiome of premature babies can have a negative impact on neurological development, and Eugene Chang found mouse pups whose mothers were treated with antibiotics were more likely to develop inflammatory bowel disease.
Constantly treating individuals with antibiotics is not a realistic scenario, even for those with genetic predisposition for Alzheimer’s, but Sisodia is keen to investigate further. He has recently been awarded a grant of over $2,000,000 to continue his research into Alzheimer’s disease and immunity. The money comes from the Good Ventures project, involving Massachusetts General Hospital, University of Southern California, Northwestern University and Washington University, with total funding over $10,000,000. The hope is this collaboration can uncover mechanisms at play between our microbiome, our immune system, and Alzheimer’s disease.
This is just another example of how the microbiome offers keys for exploring new preventative and treatment approaches for healthy longevity. As Bette Davis once suggested, “Old age ain’t no place for sissies,” but maybe someday losing our mental fitness may not top our list of concerns about aging.
Helen Robertson is a postdoctoral scholar in Molecular Evolutionary Biology at the University of Chicago, with a keen interest in science communication and science in society.

Nov 15, 2019 | Immunology, Microbiome, Neuroscience
by Elise Wachspress
Celiac disease is a serious autoimmune disorder. When those with celiac eat gluten—a group of proteins found in cereal grains like wheat and barley—their immune systems respond by inflaming and damaging the little “fingers” of tissue that absorb food nutrients in the small intestine.
About one in every 100 Americans has celiac, but many don’t realize it. The disease can be hard to diagnose, because symptoms are so diffuse: anemia, osteoporosis, loss of dental enamel, heartburn, headaches, tingling hands, joint pain, a blistery skin rash, etc. Children may suffer vomiting, diarrhea, poor appetite, muscle wasting, and even failure to thrive; adolescents may be abnormally small for their age, with delayed puberty.
Among the hardest symptoms to pinpoint and link to celiac is what some patients call “brain fog.” Those with the disease often report episodes of headaches, depression, moodiness, difficulty concentrating, fumbling to choose words, and/or feeling tired even though they just got out of bed. Sometimes only when people are diagnosed with celiac, change to a gluten-free diet, and then find these symptoms disappear do they realize how celiac inflammation affected the clarity of their neural processing.
The problem is, total gluten elimination is hard to accomplish. While gluten-free foods and restaurants are becoming increasingly common, food is fundamental to most social relationships, and it’s hard to manage every interaction without seeming prickly or oversensitive.
And many “non-food” products use gluten as an edible “glue” to bind mixtures together, including some vitamins, medications, lipsticks and lip balms, even bouillon cubes. Then there are the products one might never suspect involve gluten, like pickles, hot cocoa mix (Celiac patients often make their own), and soy sauce (One can substitute the safer tamari).
So what happens when a patient with celiac has an inadvertent exposed to gluten? Or the pizza shows up in your child’s school and resistance is low? Some people find themselves living through several days when their brains just don’t seem to function. Work and school become a challenge, even for people who are normally bright and creative. People accidentally exposed to gluten report symptoms from irritability to anxiety to full-blown panic attacks.
Bana Jabri, MD, PhD, has long been interested in understanding the neurological distress that sometimes follows accidental gluten exposure. She wants to find out if immune factors called cytokines, released in response to gluten exposure, affect brain chemistry and the nerve centers feeding back to the gut. Understanding the relationship would provide a better understanding not only the neurological mechanisms involved in celiac disease, but also in other autoimmune conditions, like multiple sclerosis and rheumatoid arthritis, in which patients also report similarly diffuse cognitive impairment.
Jabri has established a collaboration with Jean Decety, PhD, a UChicago neuroscientist internationally recognized for his work in using fMRI (functional magnetic resonance imaging) to understand affective behavior. While a handful of case studies have used fMRI to study extremely serious neurological symptoms in individual patients with celiac disease, no one has yet undertaken a larger study of how celiac creates the “brain fog” that seems such a common complaint.
The plan is to have patients undergo fMRI, immunological, and other testing before and after a controlled gluten ingestion, to map the changes in all these factors. Jabri and Decety hope the results will help generate novel insights into the neurological impact of the disease and potential therapeutic avenues to prevent these negative outcomes.
Right now they are searching for funding to support these studies. But what they find may make life a lot easier for the three million Americans living with celiac disease, some living in fear that they may accidentally ingest something that will put them in a fog for days.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office

May 29, 2019 | Microbiome, Neuroscience, News Roundup
A selection of health news from the University of Chicago and around the globe curated just for you.
Improving care for young hearts
Ivan Moskowitz is investigating the genetic causes of pediatric congenital heart disease (CHD) in an effort to improve diagnosis and treatment of children born with this condition. A recent gift from The Heart of a Child Foundation will help support his research. (Give to Medicine)
Antibiotic treatment alleviates Alzheimer’s disease symptoms in male mice
Researchers at the University of Chicago have demonstrated that the type of bacteria living in the gut can influence the development of Alzheimer’s disease symptoms in mice. Sangram Sisodia featured. (UChicago Medicine)
Addressing social needs and structural inequities to reduce health disparities
“Entering Asian American and Pacific Islander Heritage Month, a cutting-edge issue is addressing social determinants of health, which are especially critical among diverse Asian American ethnic groups that vary in education, income, and acculturation,” writes UChicago Medicine’s Marshall Chin. (NIMHD Insights)
Phage therapy to prevent cholera infections—and possibly those caused by other deadly bacteria
Discovered a little more than 100 years ago, bacteriophages, or phages, are generating renewed interest as potential weapons to fight bacteria that are resistant to multiple antibiotics. (The Conversation)
Common food additive found to affect gut microbiota
Experts call for better regulation of a common additive in foods and medicine, as research reveals it can impact the gut microbiota and contribute to inflammation in the colon, which could trigger diseases such as inflammatory bowel diseases and colorectal cancer. (ScienceDaily)
Mar 4, 2019 | Neuroscience
by Folabomi Oladosu, PhD
Post-doctoral researcher specializing in pain and women’s health at NorthShore University HealthSystem
Every now and again, you wake up to find your arm or leg didn’t quite wake up with you. You dread it, but you wiggle through the pricking, tingling sensations until your once-numb limb is ready to start the day. What if, despite your best efforts, the numbness persists? Numb tingling limbs are one of several hallmark symptoms associated with multiple sclerosis (MS), an autoimmune disorder where the immune system attacks the central nervous system.
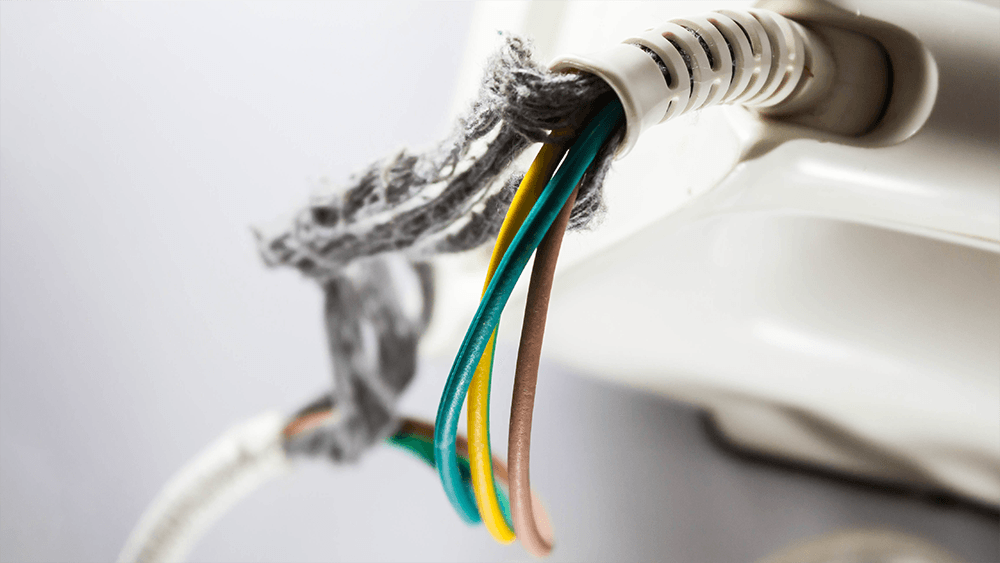
Our nerves are somewhat similar to an electrical power cable. The inside of a nerve is made of smaller nerve fibers. These fibers conduct important electrical signals and messages throughout the body. The outside of the fibers are covered by myelin, a fatty material formed by specialized brain cells. Thanks to its insulating properties, myelin speeds the transmission of electrical messages throughout the body.
If you bend any cable too often, eventually the outer plastic coating starts to fray, exposing the inner metal wiring. The cable may still work, but over time, it becomes less and less reliable (raise your hand if you’re on your third phone power cable). A similar phenomenon occurs in MS. The immune system attacks the body’s own cables, damaging the specialized cells and degrading the insulating myelin on nerve fibers. Over time, this demyelination produces a range of symptoms, including fatigue, depression, pain, and movement issues.
First-line treatments for MS aim to dampen the immune system to reduce demyelination. Although effective, these treatments make the body vulnerable to sickness and infection.
What if there was a way to directly protect the cells and the myelin they produce? Brian Popko and his team wanted to know if Sephin1, a small molecule that enhances a cell’s defensive response to environmental stressors, could protect nerve fibers from demyelination.
Popko uses a mouse model where an injection of myelin proteins coupled with a toxin generates an immune response similar to that in MS patients, initiating a MS-like disease in mice. He and his team then injected the mice with Sephin1 and followed the mice’s responses for 35 days. In this mouse model, clinical symptoms usually appear after 11 days and peak on day 17. When given one week after the initial injection to create MS-like symptoms, Sephin1 delayed disease progression, with clinical symptoms peaking on day 26.
The findings suggest that Sephin1 delayed disease progression by boosting the defensive response to insults from the immune system. When compared to control mice, mice that received Sephin1 had more of the specialized brain cells and less demyelination in the spinal cord on day 17. Sephin1 also dampened immune cell activity in the spinal cord.
The team went on to discover that Sephin1 worked even better when it was paired with interferon beta (IFN-β), an anti-viral protein native to the body, also used as a common treatment for MS. Mice that received both Sephin1 and IFN-β showed much slower, less severe disease progression.
This timely translational research from the Popko lab illustrates the promising therapeutic effects of Sephin1 for MS, especially when combined with IFN-β. Its demonstrated efficacy in MS mouse model indicates Sephin1 may help to protect indispensable nerve fibers from demyelination in MS patients. Dr Popko is currently exploring opportunities to assess the therapeutic safety of Sephin1 for MS patients.








