
Aug 29, 2018 | Cancer, Commercialization, Microbiome, News Roundup
A selection of health news from the University of Chicago and around the globe curated just for you.
UChicago awarded $1 million grant to study microbiome dynamics
Researchers from UChicago have received a $1 million grant from the W.M. Keck Foundation to study how the molecular activity of the microbiome changes in response to the environment. Eugene Chang and Tao Pan featured. (The Forefront)
Reliable point-of-care blood test can help prevent toxoplasmosis
A recent study, performed in Chicago and Morocco found that a novel finger-prick test can accurately predict infection with the parasite Toxoplasma gondii during pregnancy. Rima McLeod, whose broader work is reported in an earlier WellNews post, featured. (The Forefront)
‘Zombie gene’ protects against cancer—in elephants
With such enormous bodies, elephants should be particularly prone to tumors. But an ancient gene in their DNA, somehow resurrected, seems to shield the animals. Vincent Lynch featured. (New York Times)
Hospital bacteria are starting to tolerate hand sanitizer
Strains of bacteria have developed increased tolerance to the alcohols in hand sanitizers, which requires hospitals to rethink how they protect patients from drug-resistant bacteria. (Futurity)
Oxalo Therapeutics among recipients of scholarships from drug accelerator
Cydan II Inc., an orphan drug accelerator dedicated to creating therapies that impact the lives of people living with rare genetic diseases, announced it has selected four start-up organizations and companies, including Oxalo, to receive $5,000 scholarships to support innovations that impact the lives of people with rare conditions. (BusinessWire)

Jul 31, 2018 | Food Allergies, Microbiome, News Roundup, Research
A selection of health news from the University of Chicago and around the globe curated just for you.
Anglerfish and their headlamp bacteria have a crazy relationship
Researchers have sequenced and analyzed the genomes of the glowing bacteria living in the bulbs that hang off the heads of anglerfish. (Futurity)
Can a cat-poo parasite turn you into a millionaire
Scientists have discovered that people infected with toxoplasmosis are more go-getting. But that doesn’t mean we should all be trying to catch it. (The Guardian)
What the mystery of the tick-borne meat allergy could reveal
Unraveling why tick bites are suddenly causing a strange reaction in some people who eat meat could help scientists better understand how all allergies work. (The New York Times Magazine)
Could viruses attacking the microbiome be responsible for inflammatory bowel disease?
New research done in a mouse model of inflammatory bowel disease (IBD) has suggested that viruses called phages, which have the ability to infect and kill gut bacteria, may be involved in the disease. David Rubin featured. (Forbes)
Celiac disease: A look at what triggers it, possible prevention
Bana Jabri and colleagues at UChicago have found that a common, but mostly harmless, virus could trigger celiac disease. (KPRC 2 Houston)
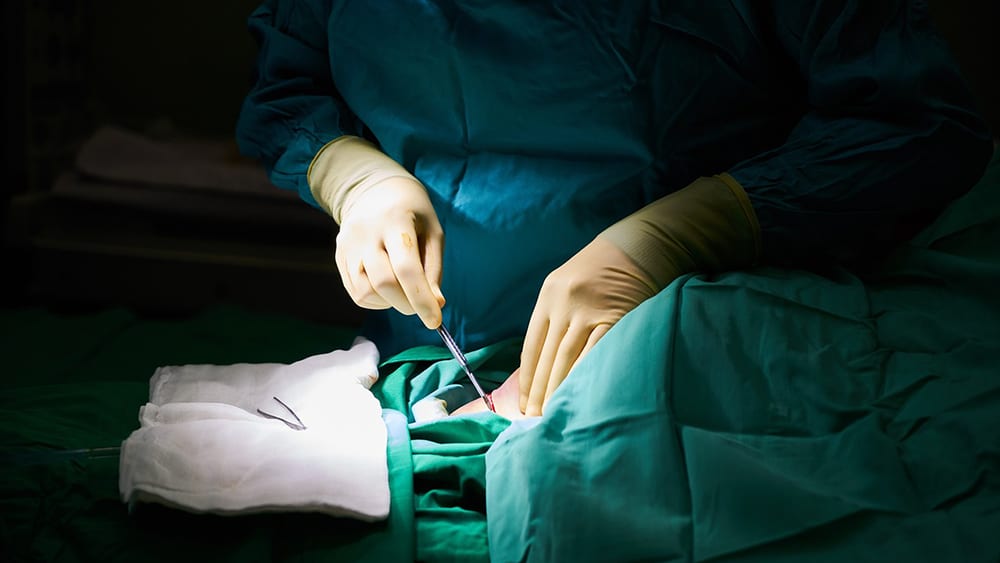
Jul 26, 2018 | Microbiome, Research, Surgery
by Claire Stevenson
Graduate student in the Committee on Development, Regeneration and Stem Cell Biology
John Alverdy, MD, is a gastrointestinal surgeon and researcher studying the mechanisms by which patients develop infections after surgery and figuring out how best to treat—and prevent—them.
“Research suggests that applying microbiome medicine … to surgical patients will change everything.” Alverdy offered that assessment recently on receiving the Flance-Karl Award for seminal contributions to the field of surgical science. The award was presented at the 103rd Clinical Congress of the American Surgical Association, the most prestigious society in the world aimed at keeping physicians up to date in the field.
Surgical site infections can be debilitating and life-threatening, increasing the risk of death after surgery up to 11-fold, even with advances in medical techniques. Alverdy has committed a large portion of his career to understanding how these infections develop following routine surgery.
He questions the assumption that all infections are caused by bacteria that enter the body during the surgical procedure. “Killing as many threatening microbes as possible prior to surgery, although a common practice, remains questionable from a scientific standpoint,” Alverdy says. His research suggests that the patient’s own microbiome, especially the microorganisms that live in each of our guts, could play a key role in these infections. Perhaps simply keeping our own resident bugs healthy and happy may actually make more sense than broadly eliminating all bacteria.
Microbes can exist in two states, a stable low-growth state and a virulent one, and they transition between these states based on cues from their environment. Alverdy reasoned that our bodies could harbor microbes that are normally innocuous, but which can switch to the harmful state when threatened, as when they have inadequate resources to support growth and reproduction.
In intestinal operations, wounds are normally colonized by gut bacteria. Alverdy and his team demonstrated that alarm signals from the patient’s own tissues and diminishing resources can shift normal bacteria to a more harmful state, significantly impairing healing. Sometimes, these normally helpful bacteria can express enzymes and toxins that cause a leak in the gut repair or other major complications.
Alverdy and his team reasoned it might be more effective to provide local resources to resident bacteria, shielding them from the patient’s alarm signals, in lieu of the traditional broad kill approach with antibiotics. By supporting the health of our normal bacteria, it might be possible to preserve them and thus keep the potentially harmful ones at bay. Alverdy’s lab is thus working to identify the “local public goods” the “good” microbes are missing in their environment and provide these directly to tissues at risk for infection.
Every individual’s microbiome is different, influenced by factors that include diet, travel, medications, and home environment. This could explain why, among patients who have been treated with the same high level of care, some develop infections while others do not. According to Alverdy, “It is time we recognize that any given individual’s disease is biologically unique,” and that, “a one-size-fits all approach to preventing infectious-related complications in surgery … will always be insufficient.”
Work like Alverdy’s, on the microbiome’s role in surgical infections, has the potential to revolutionize patient care. According to him, this could change, “the way we diagnose our patients, our approach to preparing them for surgery, the way we operate, and the way we feed and rehabilitate them postoperatively.” This could even lead to individualized medicine, where each patient’s microbiome is tested prior to surgery and their care is adjusted appropriately. The Duchossois Family Institute’s investment in microbiome research will be a part of turning this vision into a reality.

Jul 18, 2018 | Microbiome, Research
by Bethany Hubbard
What we leave behind tells a story, and Jack Gilbert, PhD, is an expert at reconstructing the narrative.
“When you poop, you poop gold in terms of valuable information about what’s going on inside your gut,” said Gilbert, who is a microbial ecologist at the University of Chicago. “And every day, the information in your poop is slightly different. Each day is providing us with a different snapshot of what’s going on inside your body, and having all of that information is immensely valuable.”
Gilbert’s work on the microbiome—the unique collection of microbes that live in each person’s gut—has earned him international recognition and spurred interest in this burgeoning field of research.
Now, through a new start up called BiomeSense, Inc., Gilbert and colleagues are working to commercialize their microbiome research and revolutionize methods for collecting and synthesizing people’s microbial data. The goal: identify and characterize microbes and their behavior in order to uncover better ways to treat disease and maintain health.
“More and more, people are realizing that our microbiome content has a lot to do with our health,” said Savas Tay, PhD, company co-founder and UChicago molecular engineer. “There are all kinds of metabolic diseases, cancers, and psychiatric diseases that are related to the microbiome.”
To better collect this valuable data, Gilbert and Tay developed new technology that analyzes a person’s microbiome through the collection and analysis of, you guessed it, their poop. Tay likens this “biosensor” to a computer chip that channels fluid instead of electricity through its wires. The biosensor can be connected to a toilet, where it extracts DNA from each bacterial cell within a stool sample and regularly sends that data to the cloud.
“So, we are looking to characterize the genetic potential—the functional potential—of the microbiome in your stool every single day in a routine and automated way,” Gilbert said. “We can basically weaponize the microbiome for clinical discovery.”
By generating more data about a person’s microbiome makeup, the pair hope to help physicians tailor more holistic treatment plans that include dietary recommendations in order to maximize the impact of certain drug treatments. For example, a doctor might recommend that a person eat more of a certain fruit because it promotes the growth of a beneficial bacterium that is known to increase the efficacy of a drug they’re taking.
The biosensor can take multiple samples over an extended period of time, which is important because the microbiome is always in flux.
“The composition of your microbes—the 500 to 1,000 different species of bacteria that live inside you—doesn’t really change very much at all,” Gilbert said. “It’s just the relative proportions that do.”
So, a stool sample taken this morning may tell a completely different story from a sample collected tomorrow, or even this afternoon. The biosensors real-time data will allow doctors to revise treatment plans daily.
“You eat something, your microbiome changes. You take a drug, your microbiome changes. You travel, it changes,” Tay said. “So, just measuring it once really doesn’t tell you anything. You need to measure it regularly.”
The BiomeSense team hopes the biosensor will eventually replace current manual methods of collection, which are less than ideal. Not unexpectedly, patients are reluctant to collect their own stool samples and send them into the lab. Plus, the microbiome makeup can be altered during transport, rendering the data unreliable. BiomeSense eliminates these variables.
The team first aims to implement the biosensor in clinical trials before pursuing a consumer product. “In the short term, we’re producing data that can be used to improve how we design drugs and improve the success of drug trials,” Gilbert said.
BiomeSense was recently awarded $250,000 from the University of Chicago’s Innovation Fund and previously received $90,000 in research funding from the Duchossois Family Institute, which will be used to complete a prototype.
“Our next big step is taking it from the bench to actually installing it in a patient home so we can start collecting data,” said company CEO and co-founder Kevin Honaker. “It’s going to take a lot of funding to get there.”
In the future, Gilbert hopes to create a global microbiome database—input a patient’s disease, prescribed drugs, demographics, and environment to determine the exact diet needed to augment their microbiome.
“BiomeSense is the only platform in the world that can generate that kind of information,” Gilbert said. “Nothing else can.”
Bethany Hubbard is associate director for digital communications in the University of Chicago Medicine & Biological Sciences Development office.

Jun 29, 2018 | Big Data, Microbiome, News Roundup, Research
A selection of health news from the University of Chicago and around the globe curated just for you.
Research at Shedd Aquarium explores how environment impacts microbiome of dolphins
Research conducted at the Shedd Aquarium with UChicago revealed new details about the microbiome of Pacific white-sided dolphins at the aquarium and how it is influenced by the surrounding environment. (The Forefront)
Can a daily pill prevent kidney stones?
Hatim Hassan and other researchers at Oxalo Therapeutics are developing a drug that uses the microbiome to prevent kidney stones. (Crain’s Chicago Business)
Viruses love what we’ve done with the planet
The more of the planet humans take over, the more we inadvertently make it a viral paradise, and a dangerous place for us to live. (Quartz)
Probiotic, supplement combo extends fruit fly lifespan
A combination of probiotics and an herbal supplement called Triphala extended the lives of fruit flies by 60 percent, report researchers. (Futurity)
Students and UChicago scientists turn Wrigley Field into data lab
Lane Tech students came to Wrigley Field to help UChicago-Argonne researchers install sensors for Array of Things, a project that collects environmental data. (UChicago News)








