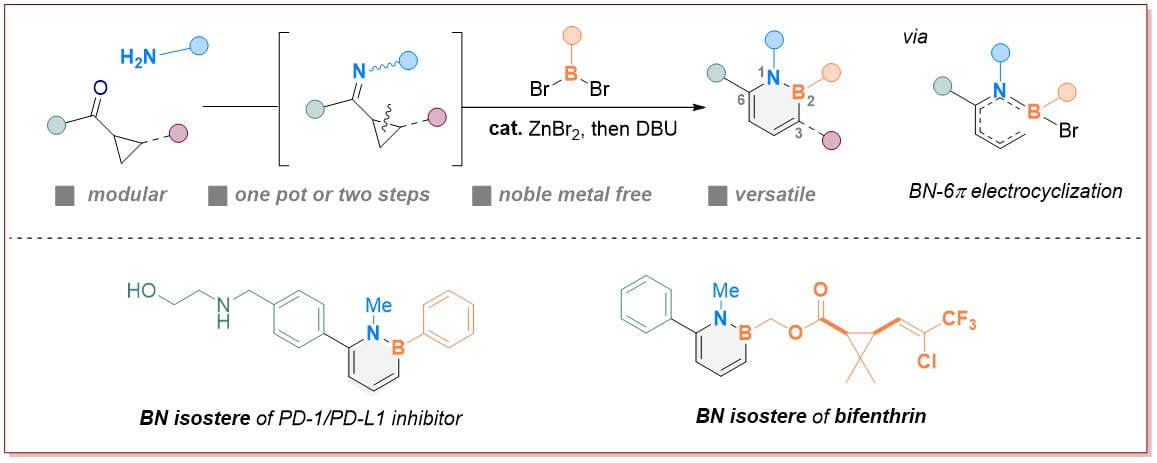
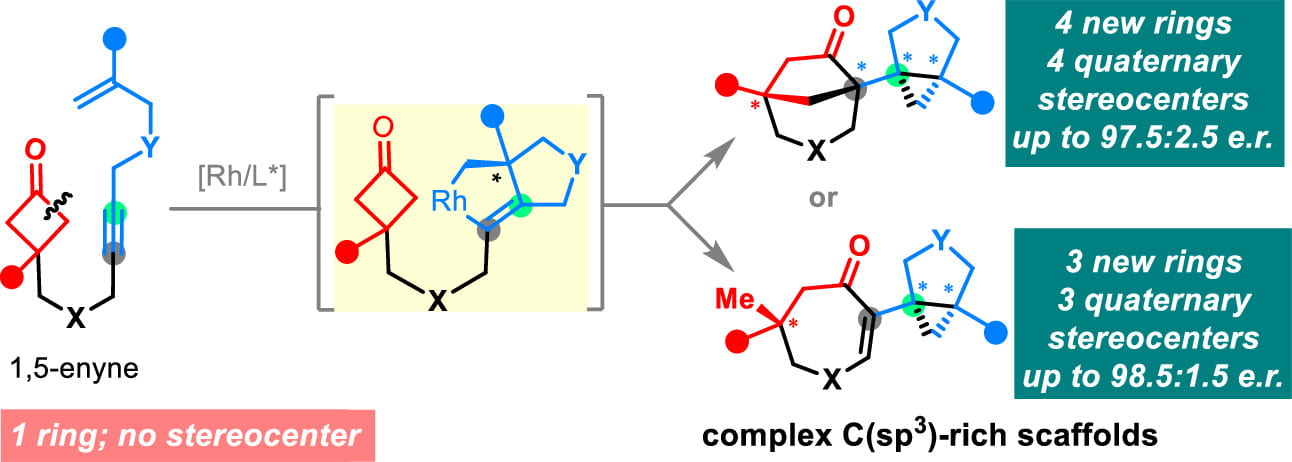
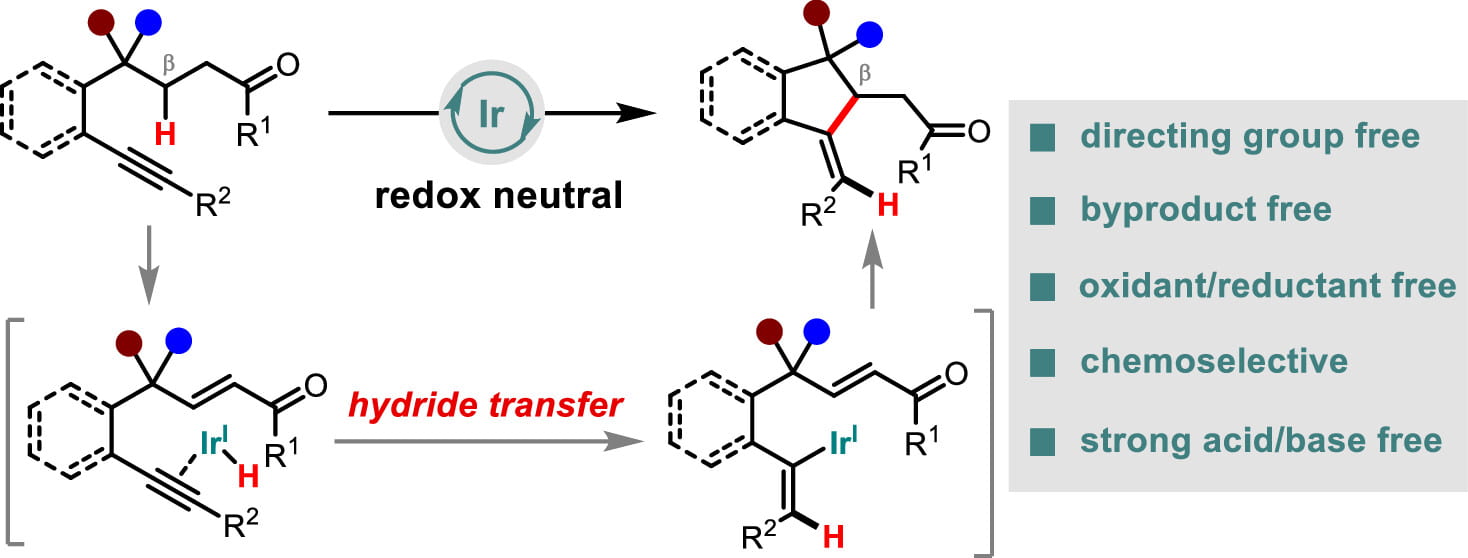
Research Directions
Our research interests is to harness the power of transition-metal catalysts to address the challenges in the arena of chemoselectivity and multi-step synthesis. Our research philosophy is guided by three questions:
1) What would be the most efficient way to introduce functional groups into (small or large) organic molecules?
2) What would be the most efficient method to synthesize various molecular skeletons (bridged or fused rings) with high complexity?
3) What would be the most efficient strategy to access mutiple structurally complicated and biologically important natural products and their analogues in a rapid fashion?

NEWS
Our research was highlighted on “Some Items of Interest to Process R&D Chemists and Engineers”!
Methyl ketones as alkyl halide surrogates: a deacylative halogenation approach for strategic functional group conversions developed by our group was highlighted on "Some Items of Interest to Process R&D Chemist and Engineers". For details, see: link
Our JACS Video Byte: Escape from Palladium: Nickle-Catalyzed Catellani Annulation is now available!
https://www.youtube.com/watch?v=t3Pe6siHWSM