
Sep 18, 2019 | Genetics, Immunology, Microbiome
In 2017, the University of Chicago Medicine established the Duchossois Family Institute: Harnessing the Microbiome and Immunity for Human Health (DFI). The institute is dedicated to developing new knowledge about human biological defense systems, including the microbiome, and their potential for preventing disease and maintaining lifelong wellness.
After a national search, renowned physician-scientist Eric G. Pamer, MD, was recruited to become DFI’s inaugural director in July 2019. Formerly with Memorial Sloan Kettering Cancer Center, Pamer is tasked with building the DFI’s research capabilities, from recruiting new faculty and building core facilities to translating discoveries into treatments that can be used in the clinic. UChicago Medicine spoke to him about his plans, and what he hopes to see the DFI accomplish.
Read the full Q&A on “The Forefront” >>

Sep 16, 2019 | Cancer, Immunology, Microbiome
by Elise Wachspress
In the world of cancer research, Thomas Gajewski, MD, PhD, is a rock star. And not just because he plays lead guitar for The Checkpoints, an all-cancer-researcher band that includes his longtime colleague Jim Allison, last year’s Nobel Prize winner for physiology or medicine (and professor at the MD Anderson Cancer Center) on harmonica.
Named a Giant of Cancer Care in 2017, Gajewski has, for more than two decades, worked in cancer immunotherapy, a field only recently recognized as the most promising for actual cancer cures—a word that makes most clinicians nervously back away.
Gajewski has dedicated his career to figuring out how to help patients’ immune systems fight cancer from within. Much of his work has focused on checkpoint inhibitors, proteins which our bodies use to keep immune responses in check, so we don’t attack beneficial bacteria or our own cells. But checkpoint inhibitors can also keep our immune system from killing cancer cells (and thus the name chosen for Gajewski’s band).
Over the past century, the idea of fighting cancers with immunotherapies has fallen in and out of favor multiple times. But oncologists are now watching the field—and Gajewski’s work—with excitement. Recent research shows that, in metastatic melanoma and many other solid cancers, immunotherapies cause tumors to regress or even disappear in 30-50% of patients. These treatments are often significantly less toxic than surgery, radiation, or traditional chemotherapy. And by training the body to recognize and kill cancer cells, immunotherapies offer an opportunity for truly durable results, offering patients a chance at living long, healthy, cancer-free lives.
But Gajewski, the AbbVie Foundation Professor of Cancer Immunotherapy, is focused past these success stories. What drives his curiosity and his passion is understanding why immunotherapies fail. And one of his most remarkable findings is that the bacteria patients carry in their guts—their microbiome—can strongly influence whether or not cancer immunotherapy works.
As with most scientific breakthroughs, this one came through a combination of dogged dedication, careful observation, and wonder. When Gajewski and his team implanted mice with melanoma tumors, they noticed immunotherapy response differed depending upon the commercial source of the animals themselves; one supplier’s mice fared much better, even though they were identical strains. The team finally found that the mice were delivered to the lab carrying a certain type of gut bacteria responded much better to the therapy.
Even more tantalizing: when the two sets of mice were housed together, outcomes improved for the mice from the other supplier. Sharing these healthy gut bacteria was key to therapeutic success.
Gajewski and team are now working to identify bacteria found in humans that can produce these effects in mice. The team will use a system in which germ-free mice are colonized with microbial material from patients, implanted with tumors, and then treated with immunotherapy drugs. The goal is to figure out the exact mechanism of how bacteria promote or restrict anti-tumor immunity—knowledge that will provide a foundation for new therapeutics. By developing computational algorithms which integrate genomic sequencing of the microbiota, the tumor oncogenes, and germline polymorphisms, they aim to get a comprehensive view of immunotherapy success versus resistance in each individual patient.
The goal is a diagnostic approach that will help clinicians—like Gajewski himself—decide the most effective way to use immunotherapy for each patient and tumor, perhaps by simply introducing new types of bacteria to a patient’s gut. While we commonly think of probiotics as a way to improve digestion or alleviate allergies, we may one day find an avenue to use much more sophisticated, designer probiotics to treat some cancers.
Gajewski recently received a promise of nearly half a million dollars from the Melanoma Research Alliance to advance this research. However, securing this award requires him to match the grant with similar funding from other sources.
For anyone ready to invest in much more effective, less debilitating treatments—perhaps even cures—for cancer, Gajewski has a strategy ready for prime time, a track record of unusual research success, and a group of musicians ready to sing about it.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office

Sep 9, 2019 | Microbiome, Transplantation
by Stephanie Folk
Organ transplants can offer patients with debilitating and deadly diseases a chance at a longer life and improved health.
But the new organs come with strings attached. Patients take on a life-long regimen of antirejection drugs, some with serious side effects, as we pointed out in a recent post.
Maria-Luisa Alegre, MD, PhD, is working to help transplant patients stay healthier longer, with fewer drugs. She is uncovering some surprising ways that diet, exercise, and microbes influence the immune system’s response to a transplant.
The immune system often attacks a new organ in much the same way it would defend against a viral or bacterial infection—a recipe for organ failure. To prevent transplant rejection, patients must take drugs to suppress the immune system, but there are consequences.
“People become more susceptible to infections and cancers when the whole immune system is suppressed,” says Alegre.
Scientists are exploring multiple strategies to improve outcomes. Alegre’s colleague Anita Chong is developing treatments designed to target only mechanisms of the immune system that react to the transplant, while maintaining its ability to fight disease. Other researchers are working to bolster tolerance, essentially retraining the immune system to treat the new organ as harmless.
Alegre and her team are approaching the challenge from yet another angle.
“In recent years my lab has become interested in the environmental factors that may influence immune response against the graft. What about dietary interventions? Things like exercise? More recently, we’ve been wondering about the microbiota.”
These are the army of microbes that colonize the body. While we often think of microbes as contributors to disease, most are actually important to health, aiding in the digestion, producing nutrients, tuning the immune system, and possibly even boosting the body’s ability to fight cancer. Alegre is using germ-free mice to test the impact of different types of microbes on transplant rejection.
“From this work we know, indeed, the microbiota influences rejection, and we have found some microbial communities that augment the immune response against a graft. But we have also found some microbial communities that can suppress the immune response.”
Alegre and colleagues are cultivating the bacteria that appear to slow down rejection to see whether they might work as a sort of probiotic therapy. They are also investigating the microbes that increase the speed of rejection in order to determine whether selectively eliminating these bugs could reverse the process.
Alegre notes that microbial influences could also help to explain why organs like lungs and intestines, which are exposed to the outside world and colonized by microbes, are typically rejected faster than organs like kidneys, which are sterile. She is exploring whether changing the microbes in a donated colonized organ like a lung could make it more like a kidney, a sterile organ, in terms of transplant tolerance.
Alegre’s studies in mice have shown that a high fat diet can also accelerate rejection of a graft, while exercise seems to slow rejection. Though the mechanism is not yet clear, the results suggest that a shot at a longer functioning transplant may be one more of the many benefits that come from staying active and eating a healthy diet.
While transplant success is a multivariable equation, Alegre’s work points out factors to improve the organ’s chances. Medical discovery takes not just a village but a giant community. By pooling their knowledge—sometimes over decades—scientists are creating a clearer picture of the complex workings of the immune system and how it responds to transplants. Their research could lead to better antirejection therapies that help patients live better, with fewer drugs.
No strings attached.
Stephanie Folk is a senior assistant director of development communications for the University of Chicago Medicine & Biological Sciences Development office.

Sep 4, 2019 | Microbiome
by Jordan Greer
Even those of us who live in cities understand the importance of forest ecosystems. They provide shelter to a vast diversity of animals and plants, generate the oxygen we breathe, and clean the air of the toxic carbon emissions we produce. But we often fail to consider the unseen, powerful forests living within our oceans.
These undersea forests are comprised of kelp, a type of algae. Algae are the world’s unsung heroes, the foundation upon which most aquatic food chains are built. They provide roughly 70 percent of the oxygen in our atmosphere. Among the algae, kelp forests have earned the nickname “blue carbon” for their superpower to remove more than 170 metric tons of the carbon released into the air each year. This function prevents ocean acidification, a process where fossil-fuel carbon collected on the ocean surface decreases the pH of seawater. As seawater pH decreases, crabs develop more slowly, scallop shells soften, and corals weaken. Kelp, with their voracious appetite for carbon, prevent this process by removing the carbon from the surrounding water and instead incorporating it into their tissues, raising the pH of the local seawater.
In seas across the globe, this blue carbon functions much in the same way as forests on land. Densely packed and able to reach heights of over 90 feet, kelp forests provide food, shelter, and protection to many marine organisms. But while their importance in our oceans is well documented, they might still be hiding their best kept secret: microbes.
 University of Chicago professor Cathy Pfister and PhD candidate Brooke Weigel recently discovered a wealth of bacteria living on the surface of kelp. Using DNA sequencing, the team was able to determine the specific groups of bacteria that live on the billowing kelp blades. Notably, two families of bacteria named Granulosicoccaceae and Hyphomonadaceae took up the most real-estate. But what do they do?
University of Chicago professor Cathy Pfister and PhD candidate Brooke Weigel recently discovered a wealth of bacteria living on the surface of kelp. Using DNA sequencing, the team was able to determine the specific groups of bacteria that live on the billowing kelp blades. Notably, two families of bacteria named Granulosicoccaceae and Hyphomonadaceae took up the most real-estate. But what do they do?
This is what Weigel aims to find out during her dissertation research. With early career funding from National Geographic, she wants to better understand the relationships between kelp, microbes, and their ocean environment. Already Weigel and Pfister have exciting ideas of what they may discover.
Remember all the carbon the kelp removed from the atmosphere? Well, Weigel found that roughly 20% of that absorbed carbon is released back into the water as dissolved carbohydrates, like sugar. The most prevalent bacteria have been reported to consume sugar for energy and, as Weigel puts it, “if you are a microbe sitting on the slimy surface on the kelp, you’re basically hitching a ride and getting bombarded with free sugar.” But this feeding frenzy may also work to the kelp’s benefit, as the bacteria may be converting essential nitrogen into a form that kelp can more easily absorb, which in turn could fuel kelp growth. So, similar to how soil bacteria help land plants take in nitrogen, these microbes may be crucial for both nutrient cycling and kelp survival.
To support this idea, the Pfister lab, in conjunction with Jessica Mark Welch, has preliminary results that show that a declining kelp population in Puget Sound also has a severely reduced community of bacteria. While they cannot prove the cause yet, they know the water temperature in Puget Sound—much warmer than in the Pacific Ocean—is one of many potential stressors for the kelp and its microbes. Further research can help determine which came first, the unhealthy kelp forest or bacterial loss, and how these observations may be connected.
As global sea temperatures continue to rise, kelp forests are slowly pushing northward. But how warm can an ocean become before it’s too warm everywhere? By investigating the microbiome of kelp forests, we may be able to answer this question and more fully understand the role of microbes in kelp declines. Losing our kelp would mean losing one of the most important forest systems on our planet, one which provides us oxygen, cleans our oceans, and creates homes for countless ocean species.
Photos by Brooke Weigel
Jordan Greer is an Evolutionary Biology graduate student and science communication intern from the University of Chicago

Aug 22, 2019 | Genetics, Microbiome
by Jordan Greer
Imagine feeling that simple cold, jog, or even a garden stroll could turn into a life-threatening event. Against your will your breath becomes labored and each inhalation becomes shorter until you feel like you are being choked by your own body.
This is a common fear for those suffering from asthma, which has claimed the lives of many. And while the effects of asthma are widespread, the African-American community continues to be disproportionately affected. According to the University of Illinois Community Assessment of Needs 2016, the percent of African-American asthmatic patients with asthma in Chicago is nearly triple that of their white counterparts and twice the US average.
To combat this disparity, the UChicago Medicine Urban Health Initiative and its collaborators recently launched the Asthma Resource Line, 1-833-3ASTHMA (1-833-327-8462). This toll-free number links community members with caregivers to create an informed dialogue about asthma-related community resources. Brenda Battle, vice president of the UHI, says the line provides both “a one-stop, convenient, and reliable source of information and a pathway for children in the community to get access to the care they need.” The South Side Pediatric Asthma Center operates the line to increase support, especially for those in the University’s neighborhood, the South Side, where there is a disproportionately higher rate of asthma diagnoses.
But what actually causes asthma? On one hand, air quality has been implicated in both initiating the disease and triggering attacks, with smog and vehicle exhaust identified as major culprits. These issues have an even more pronounced effect in Chicago, which the American Lung Association ranks as the 18th most ozone polluted city in the US, with an “F” rating for air quality.
While environment certainly plays a role in asthma, DNA is also a key player in predisposing the Black and Latinx communities to higher rates of the disease. To better understand the genetic factors that lead to asthma both in childhood and later in life, UChicago researchers Carole Ober, PhD, Hae Kyung Im, PhD, Milton Pividori, PhD, and Nathan Schoettler, MD, PhD, recently conducted the largest asthma-related genome-wide association study of childhood-onset asthma and adult-onset asthma. By comparing differences in the DNA of asthma subjects to controls without asthma, they could find genetic variations that may contribute to the onset of these conditions.
They analyzed the genetic data of over 300,000 people from a UK biobank–a repository for biological samples and clinical data. Using this massive data set, Ober’s team could elucidate which genes were associated with early and late-onset asthma.
Their results suggest that while both childhood and adult asthma share an immunologic component, childhood asthma is more heavily influenced by genes involved in other common childhood allergic diseases, like eczema and food allergies. Adult-onset asthma, on the other hand, is more likely to be triggered by environmental factors. Ober and her team’s work thus provides insights to possible genetic pathways that can eventually lead to treatments better tailored to children with asthma, while also suggesting that adult asthma and its complications may be improved by reducing exposure to triggering environmental factors.
Other UChicago studies are looking at how the microbiome, the community of microscopic organisms that live on or within us, plays a role in asthma development. The lungs, much like our skin, gut, and mouths, have their own specific communities of bacteria and fungi which may change in response to disease and indeed make a disease worse.
Steve White, MD and his team have helped confirm that patients with asthma have unique assemblages of microbes residing within their airways. They took swabs of the bronchi, the passages that lead into the lungs, and sequenced the DNA of microbes present in both asthmatic and non-asthmatic patients. Not only did they find evidence for unique bacterial and fungal communities within the airways of asthma patients, but also that the fungi present could help predict the patient’s type of asthma. These results could lead to the creation of biological tests that will help clinicians personalize asthma treatments for their patients.
As US asthma incidence increases (according to the Centers for Disease Control and Prevention, by 28 percent from 2001 to 2011 alone), the over 25 million people currently suffering from the disease and their loved ones can take heart from the work that UChicago is doing to address this growing problem. By tackling asthma with scientific research and community-centered support, a time may soon come where we can all breathe a little more deeply.
Jordan Greer is an Evolutionary Biology graduate student and science communication intern from the University of Chicago.
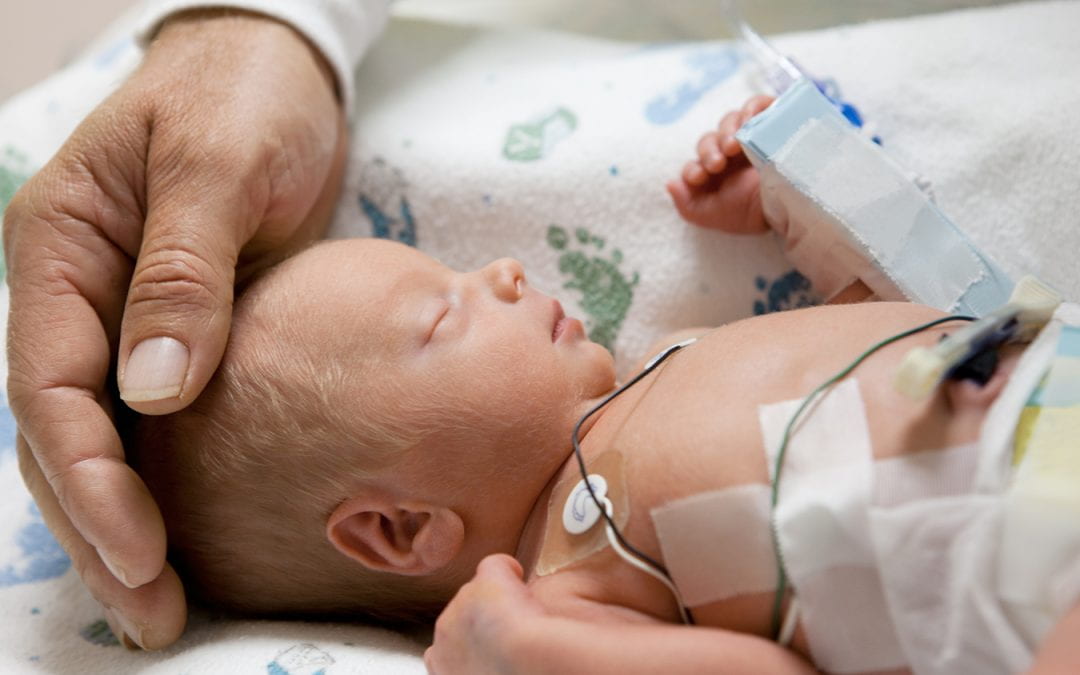
Aug 9, 2019 | Microbiome
by Elise Wachspress
Neonatologist Erika Claud, MD, has long been thoughtful about the developmental effects of the treatments she and others use to save the lives of tiny premature babies. With the help of a multi-million dollar investment from the National Institutes of Health, Claud and collaborators across the nation have been looking for ways to improve the outcomes of these babies, not just in the hospital but at home and in school, as they grow to catch up with their full-term age-mates.
Because it is common practice to treat vulnerable preemies with antibiotics, Claud was especially curious about how this approach affected the microbiome which all newborns must establish in their guts to enjoy a healthy life. Of course, one of the first benchmarks of progress for these tiny humans is weight gain, and Claud’s team has already identified microbial factors that can influence how fast these compromised babies grow.
Just as important to address—perhaps even more important—are the longer-term effects of prematurity and low birth weight, including cognitive and behavioral outcomes. Claud suspected (and the NIH found her argument compelling) that the composition of the pre-term baby’s microbiome, shaped by a hospital environment with constant instrumentation, frequent antibiotic use, multiple caregivers, and less consistent access to the microbiome of his/her own parents, might also affect brain development.
So she and her team set out to find how microbes in the gut affect the composition of fatty acids in the brain and the mechanisms of neuro-inflammation, which alters how the synaptic network, the critical electrochemical connections between nerve cells, is organized. Claud knew that neuro-inflammation could not only cause neonatal brain injuries, including cerebral palsy, but could lead to later motor and cognitive deficits, autism, even schizophrenia.
Of course, Claud and her team weren’t about to experiment on the preemies in their care, so they took microbiota from preemies experiencing poor growth and used those samples to colonize the pups of newborn mice. They also took samples from babies who were thriving and colonized other newborn pups with their bacteria, as controls.
While it appeared that differences in the microbiome did not change the fatty acid composition in the brain, the team found differences in the neurotransmission pathways. Mice with microbiota from the infants with poor postnatal growth had lower levels of specific growth factors, both circulating in their blood and in their brains. They also had higher levels of neuro-inflammation, associated with compromised synaptic organization in developing brains.
In the paper documenting their results, Claud and her team note that the first days and weeks of life constitute a critical period: “Our study characterized the effect of the early microbiome, from less than two weeks of life in preterm infants, on neuronal and myelination development in the early postnatal ages, providing direct evidence that there is a critical window in early life during which microbiota can influence brain development.” And the journal’s readers were fascinated; the editors of Scientific Reports noted that the paper was one of their most-read neuroscience stories last year.
Claud is now working with Nobel laureate and Chicago faculty member James Heckman in his long-term effort to connect cross-disciplinary experts to advance innovative approaches to human capital development research and improve individual opportunity worldwide. See Claud talk about how her research in neonatal intensive care is helping to advance the cause of Human Capital and Economic Opportunity. In this way, scientists affiliated with the Duchossois Family Institute will be improving the lives of people for decades into the future.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office










