Apr 13, 2020 | Immunology, Microbiome
by Helen Robertson
In 1933, identical twin baby boys Oskar Stohr and Jack Yufe were separated as a consequence of their parents’ divorce. Their subsequent upbringings could not have been more different: Oskar was brought up as a Catholic in Germany and became an enthusiastic member of the Hitler Youth. Jack remained in the Caribbean where they were born, was Jewish, and even lived for a time in Israel. Yet when they were reunited some fifty years after they last saw each other they had an uncanny number of similarities. They shared thought patterns, walking gait, a taste for spicy food, and perhaps most unusually, a habit of flushing the toilet before using it.
The unfortunate separation of identical twins provides scientists with an exceptional laboratory for exploring the “nature vs nurture” conundrum. How much of our identity is conferred by our genes, and how much is a product of the environment in which we are raised? This is also true for our microbiome: twin studies have shown that the microbiomes of identical siblings are far more similar than those of fraternal twins, indicating that genes are at play.
UChicago researchers Alexander Chervonsky, PhD, and Tatyana Golovkina, PhD, are particularly interested in exploring how genetics—especially the genes that control our immune system— influence the composition of our microbiome. They chose to explore this question with mice.
Both mice and humans have two types of immunity: innate, “inborn” immunity, the first line of defense against pathogens, and adaptive immunity, in which immune cells are trained by the specific pathogens they encounter to fight off the same bad guys in the future.
To make sure all the mice used in the study started with the same microbes, Chervonsky and Golovkina needed to isolate them from the regular, bacteria-filled world. A normal mouse—much like a normal human—is born into an environment with trillions of bacteria, spread to them from their mothers and cagemates, their handlers, bedding, and food. Fortunately, UChicago’s special germ-free “gnotobiotic” mouse facility allows scientists to experiment on mice born and raised in an environment that hosts precisely zero bacteria, which make the mice experimental blank slates.
The researchers transferred microbes from a source mouse, raised in a conventional environment, to several strains of germ-free mice: some genetically identical, others with slight differences in their immune-response genes. In collaboration with computational immunologist Aly A Khan, PhD, they compared the resulting microbiomes to see if changes to immune system genes resulted in different types of microbial communities.
They found that differences in the adaptive—targeted—immunity caused minimal differences in microbial composition, and that those differences affected only certain strains of bacteria. Other types of bacteria even took advantage of the genetic differences and multiplied.
The team was surprised to discover that it was the innate immune response—the one the mice were born with, that needs no training: it was more active in shaping the microbiome. But even then, the total influence over the microbes in the mice’s gut was fairly small, meaning there were likely other genetic and non-genetic factors at play in determining how bacteria colonized and proliferated in the animals guts.
The team intends to look deeper into understanding how genes and microbes influence each other in developing animals. But this study sets a valuable benchmark for future microbiome work: closely documenting how the immune system worked here in germ-free mice means comparisons against other studies are standardized. Now we have a better idea of the “nature” side of the equation.
The Gnotobiotic Research Animal Facility is a vital asset for researchers at UChicago, and just one example of the gold standard approaches being used by the Duchossois Family Institute to improve our understanding of the underlying components of health and wellness.
Helen Robertson is a postdoctoral scholar in Molecular Evolutionary Biology at the University of Chicago, with a keen interest in science communication and science in society.
Apr 2, 2020 | Immunology, Microbiome, News Roundup
Chicago doctor whose blunt speech resonated with millions has another message
In a video PSA, UChicago Associate Professor Emily Landon asks people to stay home and help flatten the curve. (NBC 5 Chicago)
Argonne National Laboratory uses supercomputers to take on coronavirus
Stephen Streiffer, deputy laboratory director for science at Argonne, discusses the use of supercomputers to combat the coronavirus. (CBS Chicago)
Here’s where bacteria live on your tongue cells
UChicago’s Marine Biological Lab scientist Jessica Mark Welch maps how bacteria are grouped together on human tongue cells. (Science News)
A new, shelf-stable film could replace needles and improve global vaccination rates
This film requires no refrigeration and can be given by mouth. (Yahoo News)
How male and female immune responses differ
Sex-specific traits of the immune system, especially in the fat cells of the body, may explain the differences between the genders in their susceptibility to certain diseases. (Futurity)
Mar 19, 2020 | Lung Disease
by Elise Wachspress
Ask just about any adult if they carry a scar, and most will admit they do. Some will point to the skinned knee that healed over badly or a rakish cleft like that in Harrison Ford’s chin; perhaps the line from a long-ago surgery or the vestige of childhood chicken pox left on an otherwise flawless décolletage. As Cormac McCarthy said, “Scars have the strange power to remind us that our past is real.”
Scars are actually webs of collagen. An injury leads to a healing response from multiple types of cells, including myofibroblasts, which manufacture the collagen that holds cells back together. Though scars may recall a painful moment in our past, they also restored our protective envelope of skin, leaving us healthy, though a little dinged.
But what if scarring becomes progressive, most often in our internal organs? In fibrotic disease, continued insults to the system lead to a runaway response, and the myofibroblasts don’t stop making collagen. The tissues involved get increasingly rigid, excess collagen starts encroaching on healthy cells, and eventually the organs involved begin to fail.
Progressive fibrosis is usually a slow process, making it hard to tell exactly what trigger or combination of triggers set the whole process in motion. This is often a particular mystery in the case of pulmonary (lung) fibrosis. With every breath, we take in everything from mold, bacteria, and viruses to environmental chemicals: which of these is the culprit? And as with allergies to substances like pet dander or peanuts, not every person responds aggressively to these factors, thus individual genetics are likely to be a factor in the disease as well.
Unfortunately, pulmonary fibrosis is fatal, and current therapies are not effective. Researchers are thus highly motivated to tackle this confoundingly complex problem from many angles. While many are particularly interested in the cell signaling that guides the process, Gokhan Mutlu, MD, and Robert Hamanaka, PhD, have been looking to starve the beast: they want to understand what nourishment myofibroblasts need to make the collagen.
Like a complex chemical plant, our bodies take in a variety of raw materials, refashion these into new chemicals, and finally manufacture the many specific compounds (proteins, lipids, nucleic acids) each of our cells need to survive and thrive. And like most chemical plants, our bodies depend on enzymes to lower the activation energy needed to get each of those chemical processes started. Mutlu and Hamanaka want to parse out the many steps in the process—from raw materials to pathological cellular outcomes—to find more than a few places to intervene in the production line and short-circuit the disease mechanism.
Knowing an amino acid called glycine makes up about a third of collagen’s structure, Mutlu, Hamanaka, and their team tried reducing this amino acid in the diets of their mouse models. But this didn’t work—it appears the fibroblasts prefer to make the glycine themselves.
They next turned to intervening in what is known as the “serine-glycine synthesis pathway,” to see if that might slow the whole fibrotic mechanism. The pathway is an important step in converting glucose—the most common carbohydrate circulating in our blood and energy source for our bodies—into the building blocks used for collagen production.
Mutlu and Hamanaka identified an enzyme crucial in the process: by using a drug to inhibit the enzyme, they could slow conversion of glucose into glycine in mice—a finding important not only in pulmonary fibrosis, but potentially in other types of organ fibrosis, like cirrhosis of the liver, as well as cancer.
Continuing to explore other parts of the collagen production process, they looked to see if they might intervene in the conversion of glutamine into proline, another amino acid essential to the collagen-making process. Proline, it turned out, also needed to be made fresh within the cells—ingesting glutamine and proline in food or as a drug did not seem to spark the collagen production. And just as with the first enzyme they identified, this second also may have relevance for many types of organ fibrosis and cancer.
This work again demonstrates how basic science—understanding how the body works and the many ways our metabolic processes can go wrong—can inform new treatments in multiple diseases.
And perhaps makes many of us grateful that our scars are evidence of the past, and not ongoing events.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office

Mar 11, 2020 | Microbiome
by Elise Wachspress
If you are a subscriber to the DFI blog (Easy! Free! See the box to the right!) or a regular reader, you’ve seen and heard a lot about mice. So much of what we have learned thus far about the interplay between the microbiome, immunity, and genetics comes from experiments with these little animals. Why?
Mice have historically been used in a lot of biomedical research. They are small and comparatively less costly to raise and house than other animals, reproduce quickly and often and have a short life span, thus studies can be conducted relatively quickly. Researchers have learned a lot about how to manipulate mice’s genetic inheritance, so the critters can make good models for some human diseases where we know the specific genes at work.
Mice have been especially useful for studying the effects of the gut microbiome, in diseases from obesity to cancer. Their intestinal track is similar to ours, with comparable anatomical features and many of the same kinds of cells—although there are some important differences.
Unlike humans, mice have a fore-stomach that holds food before digestion, creating an entry to the system much less acidic than our stomachs. Our intestines have more folds and a thicker mucus layer—an environment more friendly for bacteria looking to set up homes. The cecum, a part of the large intestine, is relatively larger in mice, offering a better place for microbial fermentation of indigestible fiber. And humans have an appendix, a place for bacteria to hang out when disaster (like diarrhea or other intestinal problems) strikes, the better to repopulate the intestines when the crisis is over.
Despite these caveats, mouse models have proven their value in gut microbiome studies, helping researchers understand some of the complex mechanisms that underlie diabetes, celiac, inflammatory bowel and others diseases. Mice offer huge practical advantages: researchers can readily control their diet, treat them with antibiotics, and transplant them with bacteria—unlike with humans, who are, none-the-less, the ultimate beneficiaries of these experiments.
Of course, transplanting mice with bacteria only works if the mice start out with either no bacteria or very well-characterized bacteria, so researchers can parse out the effects caused by the new microbial additions. After birth, the mice must continue to live their entire lives in an aseptic (no new bacteria) environment—and stay far away from others, as mice have a known habit of eating their cagemates’ poop. Managing this complicated endeavor is where Betty Theriault, DVM, and UChicago’s Gnotobiotic Research Animal Facility (GRAF) come in.
The word gnotobiotic is a mashup of two Greek roots, “known” and “alive.” The premise for the gnotobiotic facility is to understand exactly what microorganisms are alive in each mouse. The GRAF specializes in managing animals free of all organisms (bacteria, viruses, fungi and parasites) and gnotobiotic animals—those who have been intentionally inoculated with a well-defined set of microbes.
Established in 2007, the GRAF is now one of the largest and most successful academic gnotobiotic mouse facilities in the world, with over 90 flexible isolators. Theriault and her crew of 13 dedicated, full-time personnel maintain hermetically sealed, individually ventilated cages on rack systems—think sterile condominium units for mice—which assure each mouse is living with only the microbes it came in with. In 2017, the GRAF was named international laboratory animal team of the year award; you can take a three-minute tour of their approach on YouTube.
Theriault is a veterinarian with three decades of experience working with a broad spectrum of animal species in a variety of settings. After vet school at the University of California, Davis and an internship in Small Animal Medicine and Surgery at Penn, she came to UChicago to advance transplantation immunology research. Although she left for a while to pursue private practice, she returned to UChicago fifteen years ago to develop the GRAF, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, a private nonprofit that promotes the humane treatment of animals in science. (You can watch Theriault present on the challenges involved in setting up a gnotobiotic facility at the National Academies of Sciences, Engineering, and Medicine in 2017).
So what’s the future of gnotobiotic research? Studies have shown that germ-free mice successfully support complex fecal microbiota from humans in their gastrointestinal tracks. Researchers anticipate mouse models will be increasingly useful in predicting patient outcomes to therapeutic interventions or informing personalized approaches to patient care.
While knowledge gained from animal models is not always directly transferable to humans, UChicago’s Gnotobiotic Research Animal Facility is helping us understand many more of the mechanisms at work between our gut microbes and our health, providing insights that may help us live longer, healthier lives.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office
Feb 28, 2020 | Food Allergies, Immunology
A selection of health news from the University of Chicago and around the globe curated just for you.
UChicago celiac disease researchers build a better mouse
Research breakthroughs on celiac disease depend in large part on a reliably valid animal model. Bana Jabri, MD, PhD, and researchers at the Duchossois Family Institute have rigorously demonstrated the mouse model they developed is a vital tool in making progress. (Genetic Engineering & Biotechnology News)
Too many hospitalized kids get antibiotics they don’t need
Overuse poses an increasing threat to children, encouraging drug-resistant infections that are difficult or impossible to treat. Unfortunately, 25 percent of hospitalized children get inappropriate treatment. (Futurity)
Diagnosing deadly infections faster with new genomic tests
The time it takes to culture deadly bacteria and identify the right antibiotic can sometimes make the difference between life and death. Faster, more accurate identification of pathogens can save lives and reduce drug-resistant infections. (New York Times)
Benefits of getting a lung infection as a young adult?
After a young adult recovers from a lung infection, some immune cells that came to the rescue seem to stay in the lungs, protecting the patient against pneumonia in the future. (Futurity)
Janelle Monae and eating fish? UChicago doctor/chef breaks it down.
The singer/actress chalked up a scare about possible mercury poisoning to a pescatarian diet. Gastroenterologist Ed McDonald weighed in to relieve worry, with advice about the health benefits of eating fish. (Billboard)
Feb 21, 2020 | Immunology
by Elise Wachspress
Through the many posts on the WellNews blog, we’ve pointed out correlations between diet and disease, microbial diversity and wellness, sleep and neurological health. Science often progresses via experiments in which one variable is tested against one outcome, a binary approach that allows us to make definitive statements about causations—or at least correlations—between pairs of conditions.
But life—and our body systems—are messy. There are countless variables involved in keeping the human machine functioning. For starters: over 20,000 genes determine our body plan, direct the assembly of the proteins that power our cells, and lay out the immunological system that protects us from foreign intruders. Then there are the trillions of molecules in the air, water, soil, and food that we take in every day which can activate or deactivate those genes. There are the variables of temperature and sunlight, affecting our sleep cycles and how we manufacture our vitamins.
And there is the impact of the bacteria—more numerous than all our cells—with whom we share our bodies.
In this amazingly complex ecosystem, everything affects and is affected by everything else.
Recently, the world has been gob-smacked by another player in the human ecosystem: viruses. The virus COVID-19 is exerting an outsize influence on international travel, the supply chain, the economy, and the governments of China and Singapore, as well as the news media. The virus, really just a packet of molecules—not even really alive—is hard to detect until it has successfully infected cells. Now the global race is on to understand the “strategy” COVID-19 uses to get into our cells and replicate, often causing significant distress and sometimes death.
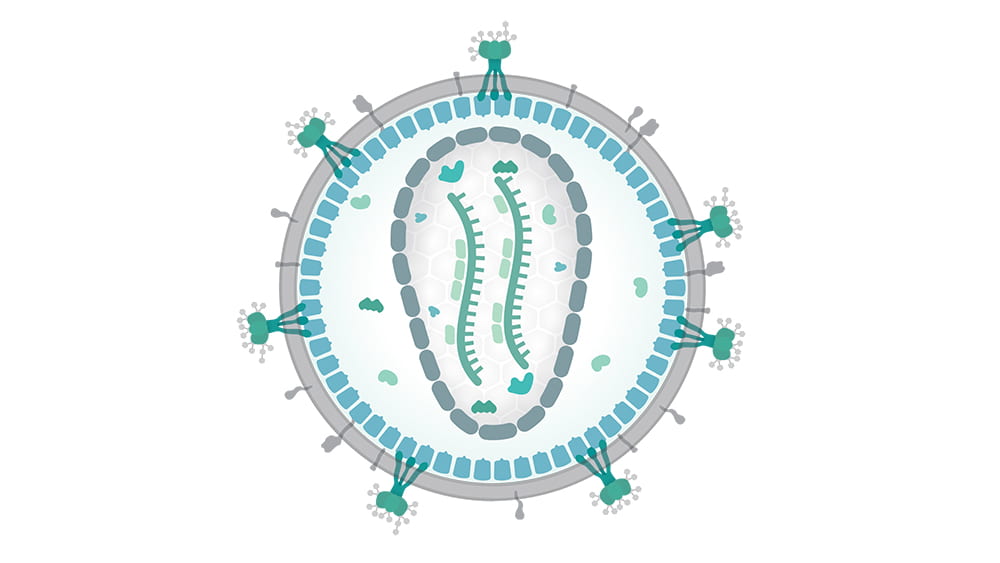
Not so long ago, another type of virus—the human immunodeficiency virus, or HIV—initiated similar havoc. HIV is a retrovirus, a packet of RNA that inserts itself directly into cell genomes, and once inserted into cell DNA, continues replicating through generations of cells.

Tatyana Golovkina, PhD
Tatyana Golovkina, PhD, is an expert in retroviruses, how the immune system detects and attempts to neutralize them, and how the retroviruses fight back. In important research nearly a decade ago, Golovkina and her team demonstrated that retroviruses use certain normal bacteria (some on the mucus membranes, others from the gut) as a kind of Trojan horse to get themselves inside the body. Once inside, the retroviruses stimulate a specific immunosuppressive protein, and with the immune system’s guard down, start invading cells and getting themselves reproduced.
Understanding how retroviruses operate is an important step in resolving multiple diseases. Some retroviruses carry cancer-causing genes in their RNA; some integrate themselves into cells primed with mutations and tip them into cancer. Other retroviruses can trigger neurological and neurodegenerative disorders.
To understand how retroviruses operate, Golovkina and her team use specialized, inbred mice that are naturally resistant to some retroviral infections. In these animals, the viruses can gain entry into the cell, but can’t get their RNA inserted into the cell’s DNA. That’s because the mouse’s cells produce a substance—an antibody—which coats the outside of the viral RNA packet, keeping its payload isolated from the cell nucleus.

Photo credit: Daniel Beyer/Wikimedia Commons. Translated by Raul654. CC BY-SA 3.0 – https://creativecommons.org/licenses/by-sa/3.0/deed.en
Golovkina’s work with these mice has already provided significant information that could help us fight retroviral infections. She has identified the single locus—the exact place in the genome—with the instructions for making the mouse’s antibody-coating factor… and it turns out that humans have an analogous gene. Her team has also found a receptor in the mouse immune system that detects the retrovirus and signals the body to make the antibody… and it just so happens that humans have an analogous mechanism, known to detect HIV. So the team has found both a potential target for treatment and an avenue for signaling that target.
More important, they’ve found this antiviral system is present and active from the day the mice are born.
Long experience has shown that babies are best vaccinated several weeks or months after birth, when their immune systems are more fully developed. But we also know that AIDS and other retroviruses can be passed from mother to newborn during birth and breastfeeding. Golovinka’s studies of how mice repel viral attacks can help devise inoculation strategies that protect these babies, as well as adults, from HIV and other retroviruses. It may also help address non retroviruses, like hepatitis, that share some similar mechanisms.
While the complexity of the human system sometimes feels overwhelming, discoveries like these and scientists like Golovkina are, step by step, providing the knowledge we need to live happier, healthier lives.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office
Main photo credit: Thomas Splettstoesser/Wikimedia Commons (CC BY-SA 4.0)
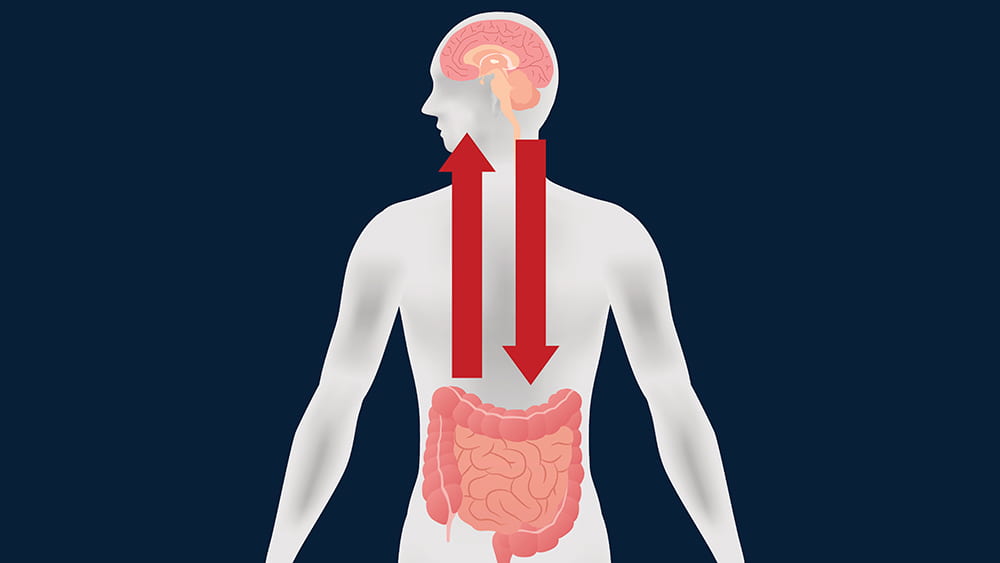
Feb 5, 2020 | Microbiome, Neuroscience
by Elise Wachspress
If you read the news—or this blog—then you know that researchers are identifying more and more interactions between the brain and the gastrointestinal tract. Some of these linkages are directly neurological; many others are chemical interactions caused by digestive byproducts of the bacteria in our guts.
A large group of scientists from the University of Chicago (led by Issam Awad, MD), the University of Pennsylvania, and universities and academic medical centers from New York to San Francisco and Australia to Germany have found another mechanism on the path from the gut to the brain—and with it, perhaps a new way to intervene in diseases that affect the most inaccessible organ of our bodies. Their report was the cover story of Science Translational Medicine this past November.
The story starts with decades of research on a rare condition, cerebral cavernous malformations (CCM). CCM causes enlarged, irregular clusters of capillaries, the tiniest blood vessels. CCM capillaries have abnormally thin walls, without the elastic fibers that make the vessels pliable. These irregularities, which make the vessels prone to lesions and blood leakage, can occur throughout the body. But they are by far most worrisome in the brain and spinal cord, where the leaks can lead to seizures, stroke, hearing or vision loss, even paralysis.
CCM is caused by genetic mutations in one of three different genes, but one of these is not like the others. Patients with mutations in the PDCD10 gene usually suffer much earlier, more severe health events, like brain hemorrhage.
A disorder with such disastrous outcomes linked so closely to single gene mutations encouraged researchers to develop mouse models for each of the three mutations. The models could help them discover the molecular mechanisms involved in each—especially what was different in the PDCD10 mice—and exploit what they learned to develop new treatments and preventive measures for CCM.
But examining the brain slices of the mice under the microscope was expensive and time-consuming. An Australian colleague noted that he had heard of instruments that could do x-ray microcomputed tomography on tiny subjects. Awad discovered only one in the entire Midwest, which happened to be in the basement of ivy-covered Culver Hall, one of the oldest buildings on the UChicago campus. Evolutionary paleobiologist Zhe-Xi Luo, PhD had acquired this microCT (now dubbed a Paleo CT!) to examine tiny, delicate insect fossils. A partnership was born, and, with Luo’s support, Awad’s team had a much faster way to assess precisely the amount of bleeding in each mouse brain and the correlation with various genetic, dietary or pharmacologic manipulations.
The investigators found that mice that developed disease did, in fact, share a characteristic microbiome. These bacteria produced a lipopolysaccharide—often called an endotoxin—on their outer membranes, a likely culprit in fostering disease progression. But the fact that the microbial communities were similar among all three genetic models indicated that it wasn’t the microbes alone that made mutant PCDC10 so much more virulent than the other two mutations.
The team moved on to test whether the PDCD10 mutation might cause some kind of rupture in the mucus membranes coating the gut, and there they found the smoking gun. The mutant gene did, in fact, attack the gut’s mucus layer, allowing the offending microbes access to the deeper tissues of the colon. With the integrity of the gastrointestinal tract compromised, bacterial byproducts could leak into the mice’s blood stream and be carried to the brain.
Awad’s team is currently extending this research in mice to patients with CCM. They hope to see how the microbiome can be used as a biomarker of disease and how targeting therapies to the gut might prevent brain bleeding. In the meantime, they think CCM patients might want to avoid polysorbate 80. They might also try any conditions or foods—or drugs—that foster mucus production, which might be helpful in preventing further degradation not only in the gut, but also the capillaries in the brain.
There are implications for the rest of us. The Awad team has found genetic anomalies in the aging brain similar to those in CCM. So what they learn about the mechanisms of this rare disease might also be used to prevent brain bleeding in the rest of us as we age. By carefully manipulating PDCD10 and the mucus layers of the gut, we may one day learn how to better care for what is arguably the most important and delicate organ in the body—the brain.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office

Jan 31, 2020 | Microbiome, News Roundup
Happy New Year! A selection of health news from the University of Chicago and around the globe curated just for you.
Five things you can do to make your microbiome healthier
Fruits and veggies, exercise, and probiotics—yes—but also add resistant starch (like beans and potatoes, especially if refrigerated after cooking) and experiment with different fibers (including whole grains, legumes, and cruciferous veggies like broccoli, kale, cabbage, cauliflower, and bok choy) to maximize your microbiome. (The Conversation)
The difference between celiac, non-celiac gluten sensitivity, and gluten allergy
UChicago pediatric gastroenterologist Ritu Verma, MD, medical director of the University of Chicago’s Celiac Disease Center, explains the characteristics and treatment of each. (The Forefront)
Human health is in the hands of bacteria
Martin Blaser of Rutgers University writes about the dangers of antibiotics overuse and makes the case for a microbiota vault, to preserve ancestral microbes for future generations. (Time)
Installing air filters in classrooms can have large educational benefits
In the face of growing proof of impact of air pollution on cognition, researchers from New York University found that installing $1,000 air filters in a Los Angeles school affected by a massive gas leak raised a class’s test scores as much as cutting class size by a third. (Vox)
Exposure to diesel exhaust particles linked to susceptibility for pneumococcal disease
Streptococcus pneumonia, a common cause of pneumonia and meningitis, usually live harmlessly in the nose and throat of healthy people, but research shows diesel particulates reduce the ability for the immune system to keep these bacteria in check. (Science Daily)

Jan 22, 2020 | Microbiome, Neuroscience
by Helen Robertson
What is your biggest concern about growing old? A decline in physical fitness? A loss of independence? Or perhaps it’s the fear that your mental fitness might start to lose its edge?
For the 50 million people worldwide living with dementia, that last scenario is a reality. A dementia diagnosis comes with big personal, social, and financial consequences: the cost of care for someone living with dementia is reportedly higher than that of both heart disease and cancer combined.
The most common cause of dementia in the US is Alzheimer’s disease. Although its symptoms are well known—cognitive decline, neuroinflammation, and the tell-tale formation of amyloid plaques, the hard aggregation of proteins between nerve cells in the brain—the precise cause remains unknown, and there is no current cure.
As the world population continues to age, dementia is increasing. The need to uncouple its complex biological processes is urgent.
Sangram Sisodia, PhD, has spent the past three decades investigating just that. But in recent years his Alzheimer’s research has taken an exciting and unexpected focus: the gut.
Thanks to recent findings, many of them by research teams at UChicago, we have learned that the bacteria living in our guts can affect many aspects of health. Normally, our gut microbiome contributes to everyday wellbeing and immunity. But just like any other community, the composition of our microbiome can fluctuate on a near-daily basis. And when a shift in balance occurs, things can go awry.
Our intestinal microbes have a particular influence on immunity and neurological function, both important factors in Alzheimer’s. Those with the disease have also been found to experience a change in the character of their gut microbiome.
That’s where Sisodia stepped in.
Over the past few years, his team has been using mouse models of Alzheimer’s to understand how the composition of our gut microbiome might influence neurological inflammation caused by certain immune cells. They thought this inflammation could contribute to both the protein deposition and neurodegeneration in Alzheimer’s.
Sisodia’s research has already generated some interesting findings. His studies, published in Scientific Reports and the Journal of Experimental Medicine, showed that the long-term treatment of mice with broad-spectrum antibiotics reduced neuroinflammation and slowed the growth of amyloid plaques.
After treatment, the mice also showed significant changes in the composition of their gut microbial communities. Some types of bacteria completely disappeared; others multiplied—suggesting bacterial diversity in the gut plays a role in the immune response during disease progression.
But only for male mice. In females, antibiotics actually increased the inflammatory response, with no change in brain plaques. With Alzheimer’s more prevalent in women than men, this gender difference in immune response clearly warrants more study.
Myles Minter, postdoctoral scholar in Sisodia’s lab who is now a research analyst at William Blair, wondered what might happen if one could prevent Alzheimer’s by treating it early—really early. He gave two-week-old mice pups antibiotics for just one week—which left lifelong effects on both their gut microbiome and amyloid plaque formation.
But this is no simple solution. UChicago neonatologist Erika Claud has shown how changes to the microbiome of premature babies can have a negative impact on neurological development, and Eugene Chang found mouse pups whose mothers were treated with antibiotics were more likely to develop inflammatory bowel disease.
Constantly treating individuals with antibiotics is not a realistic scenario, even for those with genetic predisposition for Alzheimer’s, but Sisodia is keen to investigate further. He has recently been awarded a grant of over $2,000,000 to continue his research into Alzheimer’s disease and immunity. The money comes from the Good Ventures project, involving Massachusetts General Hospital, University of Southern California, Northwestern University and Washington University, with total funding over $10,000,000. The hope is this collaboration can uncover mechanisms at play between our microbiome, our immune system, and Alzheimer’s disease.
This is just another example of how the microbiome offers keys for exploring new preventative and treatment approaches for healthy longevity. As Bette Davis once suggested, “Old age ain’t no place for sissies,” but maybe someday losing our mental fitness may not top our list of concerns about aging.
Helen Robertson is a postdoctoral scholar in Molecular Evolutionary Biology at the University of Chicago, with a keen interest in science communication and science in society.

Jan 15, 2020 | Evolution, Immunology
by Elise Wachspress
After phenomenal increases in lifespans over the past century, longevity in the US has begun to head in the wrong direction. Since 2014, average life expectancy has ticked downward, from a high of 78.9 years to 78.6 last year—placing us 37th among the world’s countries, tied with Albania and more than five years behind long-lived Japan (83.7 years).
The American Academy for Family Medicine cites several causes: the opioid epidemic, increasing suicide rate, and a rise in maternal mortality. Behind these specific threats lurks a shadowy but more endemic source of reduced life expectancy: growing inequality.
As long ago as the first century, the natural philosopher Pliny the Elder recognized a connection between socioeconomic status and lifespan, citing kings, senators, consuls, priests, and performers who lived to a remarkably old age. Some reasons for the perceived disparity seem pretty obvious: lack of food, shelter, hygiene, and medical care have put poor and less educated people at a disadvantage. But the data show that social status also significantly predicts longevity. For instance, despite war and the treacherous travel involved in their diplomatic missions, our first three presidents lived to ages 67, 90, and 83 respectively, when the average US resident could expect to reach only 37. And a survey of the Academy Award nominees shows that those who won the Oscars lived four years longer than those who didn’t.
How does social adversity get inside our skin? One theory is that low social status revs up genes that control the immune response, perhaps preparing lower-status members of a community to fight infections—but at the same time making them more vulnerable to conditions caused or exacerbated by inflammation, like heart disease, cancer, diabetes, and many others.
To investigate how status might influence the immune system, a group of scientists from the US and Canada studied a population of 45 rhesus monkeys, a highly hierarchical species relatively close to us on the evolutionary “tree.” They divided the monkeys into nine groups of five, observed them over a year as rank was established within each group, then re-sorted them into new groups, placing those of similar social rank together—thus forcing an abrupt change in the individual rank of most of the monkeys.
To see how the change in social status affected the animals’ immune states, the researchers took blood samples from each. They found that the lower the social status of each monkey, the stronger was the inflammatory response engaged by their immune system.
The researchers’ clever experimental design also allowed them to see how past social status affected the monkeys’ later immune states. The researchers found that animals at the bottom of the pecking order in the first year of the study still showed immunological traces of past low status, despite their improved social position in their second communities. And though the correlation was weaker, those who enjoyed high rank the first year also suffered immunologically when resorting forced them into a lower social position.
So no matter how each of these primates started in life, being at the bottom of the social ladder was demonstrably detrimental to their immunological state, even with sufficient food, housing, and care.
Research like this is a good example of how the Duchossois Family Institute (DFI), focused on understanding how to maximize health for the greatest number of people, can help us think more deeply and productively about wellness and what it will take for all to thrive.
This work is also an illustration of the approach to training and collaboration central to the organization of the DFI. The lead authors of the paper, Jenny Tung, PhD, and Luis Barreiro, PhD, both did critical postdoctoral training at UChicago early in their still-youthful careers—much like the cross-disciplinary postdocs that will now be supported through the DFI. Though Tung (a 2019 MacArthur Fellow) is now on faculty at Duke University and Barreiro (after an appointment at the University of Montreal) is now a professor at UChicago, they continue to collaborate closely and productively.
And building on that training and collaborative spirit, they are now leading a new generation of graduate and postdoctoral students to uncover how scientific research can improve health for many across the world.
Elise Wachspress is a senior communications strategist for the University of Chicago Medicine & Biological Sciences Development office.