How did we determine starting voltages?
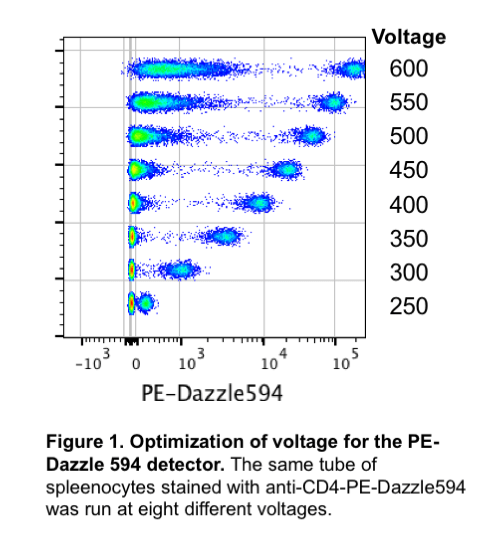 Each detector on each instrument has a “sweet spot” or optimal setting. In order to determine these optimal settings, we ran the same tube stained with one antibody at 6 different voltages (Figure 1). This voltration test clearly shows what happens when the voltage changes: at lower voltages it may be difficult to resolve the positive population from the negative population, but at higher voltages the negative population begins to spread out and/or the positive population is off the plot (saturating the detector). We can assess these different voltages numerically by calculating the separation index or staining index (SI). The SI takes into account the distance between the means of the positive and negative populations as well as the spread of the negative population. Ideally, we want to use a voltage where we have the highest SI. Figure 2 demonstrates that increasing the voltage does increase the SI – to a point. Eventually, the SI will plateau and increasing the voltage will not improve the data.
Each detector on each instrument has a “sweet spot” or optimal setting. In order to determine these optimal settings, we ran the same tube stained with one antibody at 6 different voltages (Figure 1). This voltration test clearly shows what happens when the voltage changes: at lower voltages it may be difficult to resolve the positive population from the negative population, but at higher voltages the negative population begins to spread out and/or the positive population is off the plot (saturating the detector). We can assess these different voltages numerically by calculating the separation index or staining index (SI). The SI takes into account the distance between the means of the positive and negative populations as well as the spread of the negative population. Ideally, we want to use a voltage where we have the highest SI. Figure 2 demonstrates that increasing the voltage does increase the SI – to a point. Eventually, the SI will plateau and increasing the voltage will not improve the data.
 So how did the CAT Facility choose the optimal starting voltages? First we stained mouse spleenocytes with a single fluorophore. Using the same clone of anti-CD4 and the same concentration of antibody (0.1 ug/tube) we created single stained samples for most of our frequently used fluorophores. After running each tube at 6 different voltages, we calculated the separation index and selected the optimal voltage for each detector.
So how did the CAT Facility choose the optimal starting voltages? First we stained mouse spleenocytes with a single fluorophore. Using the same clone of anti-CD4 and the same concentration of antibody (0.1 ug/tube) we created single stained samples for most of our frequently used fluorophores. After running each tube at 6 different voltages, we calculated the separation index and selected the optimal voltage for each detector.
For other fluorophores that we did not run ourselves, we used an alternate method. BD instruments have automated software called Cytometer Setup & Tracking (CS&T) that is used to characterize, setup and track the instrument performance. One part of CS&T is determining an optimal voltage for each detector. So why did we do our own voltration test instead of just using the CS&T? CS&T beads are used to determine optimal voltages – these beads contain an unstained, dim positive and bright positive bead. The optimal voltage is then calculated by determining the highest SI for separating the dim positive bead from the negative bead. The problem with that is fluorophores that are dim require a very high (above 700) voltage to resolve the dim positive bead from the negative bead. For best results, panels should be designed so that dim fluorophores are paired with highly expressed markers. If a panel is designed properly, there should not be a need to resolve a dim population in a dim fluorophore. When comparing our voltration experiment results to the CS&T results, we had similar voltages for most flurorophores. However, for dim fluorophores, we chose to instead use a voltage that optimally separated the bright positive population from the negative population.
How do you use the optimized voltages?
We suggest starting with the optimized voltages in the default new experiment. If the single stained controls are so bright that they are no longer on the plot (cells are on the right Y-axis), then the voltage should be lowered for that detector until all of the positive cells are within the plot. Increasing the voltage above the recommended voltages is not beneficial, as shown in Figure 1.
Where can you find our data?
All of our results are posted on the resources page under “Benchtop Analyzer Voltages and Staining Indexes”.
- Summary of data
- Full data


I am working on immunophenotyping human whole blood samples.
with three panels include 10 Antibodies in each panel.
As I am new to this field and I could not adjust any voltage setting (Just move everything towards the negative site using just an unstained tube, not adjust any voltage using a single color tube)or I couldn’t set any staining titration of antibodies.
Now after running several experiments I came across that different acquisition setting (Currently, I am using all 7 antibodies in each panel as 5 uL which suggested in datasheets from the companies),
I m looking for different spillovers in the unusual channels, and as the acquisition (Voltage/gain) setting changing, I am looking for different spillovers in different channels.
I need your help and guidance it will really help me to resolve the issue and I could optimize a good
the experiment setup.
1. To find out antibody concentration or to optimize voltage? which one I have to perform first and instead of whole blood if I could adjust antibody amount and voltage with the beads is it correct?
2. To set the correct voltage, can I resolve the current problem which I came to know that spilling over in different experiments in different channels in single controls with different acquisition settings.
3. After this all I will go for compensation set up do I am correct?
4. Is it necessary to put Channels on which I am not using during experiments. for example, there is nothing labeled with ECD or V660 than is it necessary to put these channels on during my 10 color experiments.
5. Can I optimize voltage with the same fluorophore but different antibodies only once. for example, CD95 PE and CD57 PE (they are not in one panel), if I optimized my volage for Only one antibody, can I used the same for another one as well.
Best Regards,
Suchita S. Jadhav
(Ph.D. Student)
Prof. Michael Firer reserach group,
Laboratory for Immunology & Cancer Biology,
Dept. Chemical engineering, Ariel university
Ariel, 40700, Israel
Hi Suchita,
You may find this video useful: https://youtu.be/wTUviuik6rw. To answer your questions:
1. Voltage is dependent on the cytometer, titration is dependent on the antibody. Voltage and antibody titration are not dependent on each other. The optimal voltage should be determined first and does not need to be done every experiment. You may find it easier to use beads to do a voltration test.
2. I’m sorry, I don’t understand the question.
3. Yes, compensation can only be done after voltages are set.
4. You can remove channels you aren’t using if you want to.
5. See my answer to #1.
Hi Laura,
I was wondering, can I stain some cells for all the markers in my panel and run the sample at different voltages or it’s important to run individual (single stain) samples to complete voltration?
Hi Elena,
The voltration experiment is completely independent of a scientific experiment. A voltration experiment should be done once to determine optimal voltages for a specific cytometer. When running a scientific experiment, you can start with the optimized voltages and make minor modifications before running all of your controls and samples on the same exact voltage settings. For compensation to be calculated correctly the controls and samples must be run at the same voltages.
Dear Laura,
Thank you for the excellents anwers and discussion. I will use an extense painel with 15 fluorochromes. The majotiry will be purchased from other vendor, distinct from BD. Can I use CS&T without problem? And I need to perform specific voltration for these distinct fluorochromes? Additionally, can I make voltration with my antibodies, or should I use the same antigen, as CD4, to distinct fluorochromes?
Thank you,
Eugênia.
Hi Eugênia,
Good question! Let me untangle a few things. Firstly, there is no relationship between the provenance of your antibodies and using CST, you won’t have any issues running CST for your experiment. Secondly, CST is the QC protocol for BD platforms which includes a characterization of the detectors on the instrument through voltration – using special calibration beads from the CST kit. The software identifies the optimal voltage value that separates the beads in each channels. You can do a similar experiment with CD4 stained cells or with your own samples and you should find the same values, more or less. In our hands, CST from the DIVA software gives us values that are higher than needed, but are still workable for most assays. Running a voltration experiment with your own samples – or using CD4 stained cells – will get you settings that will, if anything, make your data look better.
Let’s keep in mind that the point of the voltration is to identify the threshold point below which the system has not yet fully optimized the separation between the autofluorescence and fluorophore signals. If you increase the voltage beyond that point, you will impact a whole set of features such as your compensation matrix and the spillover spread. But it will not impact the separation of your two fractions. This is what is demonstrated in the deck of slides The Impact of Adjusting PMT Voltages on Spillover and Compensation.
So in short:
1 – you will be able to use CST, and you should definitively run it to make sure your instrument is working at peak efficiency.
2 – Running a voltration with your own samples or CD4 stained cells might help providing data that looks somewhat nicer, but the CST settings from the DIVA software will likely work as well. The main idea is: as long as your voltage is above the point where the autofluorescence and fluorophore signals are optimally separated, your data will be usable.
Lastly, just make sure that all of your signals are on scale, and none of them are so bright that the positive fraction is lost in the top bin of your axis. Then you’ll reduce the voltage and get the cells back on scale.
I hope this helps, let me know if you need further clarifications.
Hi Laura,
First, I wanted to thank you and your colleagues, I find the articles of your facility very helpful. I have been trying to understand the best way to adjust the voltages on my experiment. If I understood well the voltration is done to find out the optimal starting voltages. My question is, how do we decide, while creating a new experiment, if these voltages are actually the right ones for our sample (what I mean is that different type of samples may have very different levels of autofluorescence)? If you were to start a multicolor experiment, would you do a voltage walk for those specific antibody-fluorochrome conjugates used in the final experiment or just adjust the starting voltages to what visually looks the best?
Thank you in advance for your time!
Hi Blerina,
Laura is now working for Cytek, so I’ll try to answer you question. This blogpost describes the behavior of the detectors as we change the voltage values (or gain – depending on the type of detector being used). The key thing you should take away is that the is a fairly large set of voltage values that will work for you. It simply needs to be high enough that you get enough electrons in the system to allow the resolution of your positive fraction from the cell autofluorescence, without being so high that the the positive fraction falls off-scale. The voltage selection method described here is a simple way to get you within that range.
I read your question in two ways, so I’ll answer for both. First, you may be asking wether you need to run a voltration for every new experiment. That would be the recommended way of preparing for your experiment by experts in the field. I personally think this is something that you’ll want to consider if you are using a fairly complex panel (10+ markers) on a traditional flow cytometer (i.e. non-spectral) such as the LSR platform from BD (including the new Symphony instruments, they are all pretty much the same). The issue here will be to make sure that all of your markers are working properly on your cells and that you can eventually resolve your clusters. Otherwise, the voltration data will not be all that useful. I don’t think the voltration will provide such a great advantage if you are using a simpler panel. For some instruments the voltration seems entirely unnecessary, such as the Novocyte platform (Quanteon, Penteon), or downright cumbersome with any spectral flow instrument.
If you question is more about the level of autofluorescence of your cells and the impact the voltage may have on the resolution. Here, I’d simply say that if you can’t resolve you clusters even when you are in that range of voltages that should allow you to do so, the alternatives will include modifying your panel to pair this specific marker to a brighter fluorophore, or use the autofluorescence extraction feature on a spectral flow cytometer.
I hope this helps, let me know if I need to clarify anything.
Cheers!
David Leclerc
Thanks for this great explainer!
Is there a strong reason to use cells (e.g. splenocytes) for voltration instead of compensation beads?
Or are the results from using compensation beads for the voltration similar to using cells ?
No, the results should be roughly the same. Comp beads can be expensive though, so I don’t like using them for that purpose. On multiple platforms, QC beads are used so you don’t need to bother with staining. You’ll get some minor variations as the ‘optimal value’ between a voltration done on cells or beads, but it should not impact the quality of data and still works well enough for most experiments. The caveat I give my users is that if you’re trying to optimize the voltages for your specific experiment, I’d use your own cells. It will be beneficial if your panel is complex enough. But that’s also when I recommend moving to spectral where the gains are mostly set up already…
This might be a dumb question but when performing a voltration what do you set the voltages of the channels you’re not assessing? For example, I went on a cytoflex and I think the person before me went wild changing the voltages. I put on FITC and it was showing the highest peak in the PE-cy7 channel. If I just found the optimal voltages for FITC with all the other voltages out of whack wouldn’t it effect my optimal voltages? So my question is do you just set up an experiment for each individual fluorophore so you’re only looking at the voltage for one color during a voltration? Or do you just run CST, hope that it’s good enough for most of the fluorophores and then start titrating all of the voltages?
Hi TC,
Not a dumb question at all. The thing is that we don’t care about the other detectors when it comes to voltration. All that we are trying to do is to find a sweet spot where the voltages provide an optimal resolution for that specific detector. So when you run an experiment, you can just use the voltration result values and acquire your data. The compensation values may be somewhat high for some of the fluorophores, but it won’t have an impact on your actual ability to resolve the markers.
The CST protocol does include the voltration, so you shouldn’t have to revisit afterwards. Although I do thing values that come off the CST process are higher than what I’d normally use, probably because the beads can react differently than actual stained cells. But the values should be high enough with CST that resolution is still very good.