Fortessa and Aurora
Pritzker School of Molecular Engineering InstrumentsHome » Benchtop Analyzers » Pritzker School of Molecular Engineering Instruments
Overview
The CAT Facility manages the flow cytometry equipment located in the PME research space. Non-PME users should book instruments located in the main lab primarily. Access to the William Eckhardt Research center requires proper ID card authorization.
PME Instruments
Model : Aurora
Manufacturer: Cytek Biosciences
- 5 lasers, 64 fluorophore detectors, 3 FSC/SSC detectors
- Detect a wide variety of fluorescent antibodies/dyes/proteins
- High sensitivity
- Cope with autofluorescence with the autofluorescence extraction feature
- Load samples from standard FACS tubes or a variety of plates
- Volumetric counter allows for cell count
Fortessa Sample Preparation Tips
Accepted Tubes
All three LSR/Fortessas require a specific 5mL round bottom tube (Falcon #352008). For the HTS a variety of plates are accepted.
Sample Volume
If you are new to flow cytometry, it is safer to resuspend your cells (especially controls) in a larger volume. This will allow you to properly set the instrument settings without running out of sample. As you become more comfortable with the cytometer, you can use more concentrated controls so that they run faster on the instrument.
- 150 µL absolute minimum, 300-500 µL minimum for new users, 150-300 µL for advanced users
- It’s better to bring concentrated samples, once you are at the cytometer you can easily dilute your tubes if they are running to quickly
- In general, run samples at less than 35,000 events/sec
Fortessa Resources
There is a lot of information available in the Resources Library (located under the Resources menu)! The ones relevant to the Fortessas/LSRII can also be found in the module below.
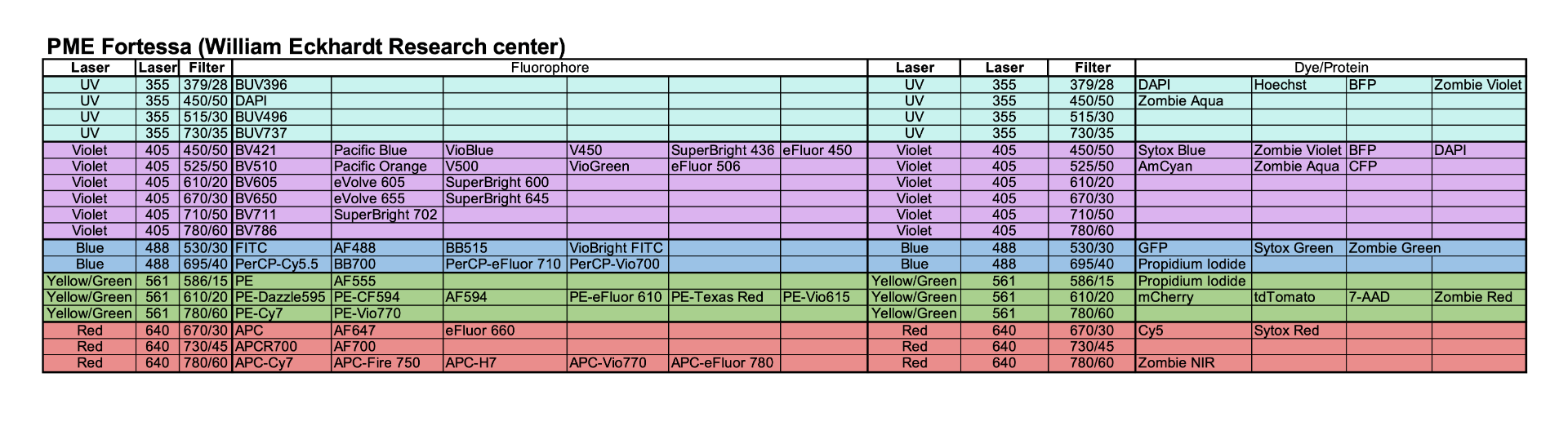
Fortessa Configuration and Suggested Fluorophores
When designing a panel, only one fluorophore per row should be used – it is not possible to distinguish fluorophores/dyes from others within in that same row. This is also posted on the physical cytometer to refer to the detectors used to identify each fluorophore. This table do not include a complete list of all fluorophores, only those that are commonly used.
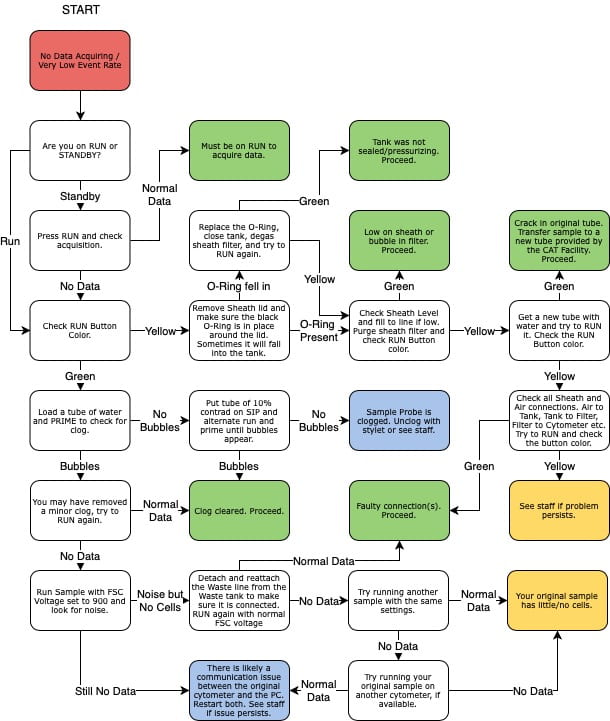
Fortessa Troubleshooting
Opening the sheath tank
The Instrument is not connecting
The DIVA software should take at most two minutes to connect to the instrument. Beyond that point, power cycle both the instrument and the computer (shut them off completely and reboot).
Very few events are showing
A few things to consider:
- Check the Run button - if it has a yellow-ish color, the sample is not being pressurized and is not flowing through the instrument. Common issues include : sheath tank is empty, the sample tube is cracked, the arm is not closing properly. Otherwise, contact the staff for assistance.
- The sample may be clumpy, filter through a 45um mesh to remove obstructing elements;
- The tube support arm may be set too high: the black hockey puck on which the tube sit on the sample injection port (SIP) can be raised or lowered by screwing it clockwise or counter-clock-wise. It may be set too high and blocking access to the sample;
- The adjustment dial on the front panel may be set too low, turn it clock-wise to increase pressure.
The instrument was running on an empty sheath tank
No data is showing
The event rate stays at 0. A few things to consider:
- There could be a communication issue between the instrument and the computer. With a tube of water on the SIP, raise the FSC voltage all the way to 1000. At that level, you should see all sorts of noise on the FSCxSSC graph. If the event rate remains at 0, there is likely a communication issue. Power cycle both the instrument and the computer to restore connection. If the event rate raise to several thousands events/sec, then the connection is fine and the problem elsewhere.
- With a tube of water on the SIP, press the Prime button. Bubbles should be coming out of the SIP during the process. If that is not the case, the SIP is likely clogged. With a tube of Contrad 10% on the SIP, press the Run and Prime buttons, alternating every half second until the clog is dislodged and bubbles are coming out of the SIP.
- If the clog persists, try the advanced unclogging option below.
- Contact the staff if neither these steps are successful.
Unclogging the SIP with a stylet
Please get the approval from the CAT Facility staff before you perform this procedure.
Shutdown and maintenance procedure
- Run 10% Contrad (red taped bottle) on High for 3-5 minutes.
- Run DI H20 (blue taped bottle) on High for 3-5 minutes.
- With the tube of DI H20 still in place, press Prime.
- After ~15 seconds of priming, the cytometer will automatically return to Standby.
- Make sure to leave the instrument in Standby Mode.
- If you are the last user of the day, turn off the cytometer with the green power button.
Data will not upload to the server
- The data should upload automatically every 30 minutes, so be sure that you gave enough time for the transfer to occur.
- Data should b exported to D:/BDExport/FCS. Make sure that you did not export your files in another location - either somewhere else on the disk, or a sub-folder of FCS Export.
- In some case communication gets lost, contact the staff and we will alert our IT manager.
Contact the staff
Email cathelp@bsd.uchicago.edu to reach the CAT Facility staff.
HTS unit maintenance
Coming soon.
Aurora Training Requirements
In order to use any cytometer in the CAT Facility without staff assistance, all users must complete the basic training outlined in the New User Checklist on the New Users page. The training for the Aurora specifically is:
- Flow Basics course (schedule online)
- Aurora training course
- Aurora hands on instrument training
- (hands on training for any other benchtop analyzers can be done at any time before you use another cytometer)
The Aurora training course covers all of the basic information needed to plan a successful Aurora experiment – creating proper controls, panel design tools, spectral unmixing, etc. Please contact Laura Johnston to schedule Aurora training.
Aurora Sample Preparation Tips
Accepted Tubes
The Auroras accept standard 5mL round bottom tubes (FACS tubes) as well as a variety of 96-well plates, including deep-well. It is possible to run 5mL FACS tubes with small volume tube inserts (AKA bullet tubes), however an extra setup step must be performed on the instrument to prevent damaging the sample introduction port (SIP).
Sample Volume
- Running samples on the Aurora is comparable to the Fortessas
- A minimum of 100 µL is reccomended, 150-300 µL is typical but use what is appropriate for the sample
- It’s best to bring concentrated samples, once you are at the cytometer you can easily dilute your tubes if they are running to quickly
- In general, run samples at less than 35,000 events/sec
Flow Rate
The volumetric counter allows for live monitoring of the sample flow rate. The estimated rates are:
- low: 15 µL/min
- medium: 30 µL/min
- high: 60 µL/min
Actual samples my vary. For example, many cell types run on low typically have rates around 9-11 µL/min.
Aurora Resources
There are many resources in available in the Resources Library (located under the Resources menu). These can also be accessed in the module below.