I’ve been looking for sterility-designed cell sorter for a while and have organized a Tyto demo for our users in 2018 and 2019. I’d like to share what I’ve learned so far.
Let me get some stuff out of the way before we start:
1 – I’m not thinking of the Tyto as a direct competitor to the Arias currently in my lab, or any other droplet-based sorters available. These will generally have better optics, more detectors and will separate more cell fractions. So while several features may look quite inferior to droplet-based cell sorters, my main interest with the Tyto is its ability to sort in sterile conditions.
2 – The main question I have regarding the Tyto is whether or not we can develop a successful business plan: can we find enough users in need of sterile sorting that will adapt to the Tyto way of preparing samples?
3 – I have no deep engineering understanding of that instrument. I’m basing my observations on what I saw during the demos and my discussions with Miltenyi reps and other Tyto owners.
4 – I did ask the Miltenyi people to fact-check my review on the inner-workings of the sorter. The data from Figure 2 was obtained during the 2019 demo in the CAT Facility and the figure was prepared by the Miltenyi Application Specialist.
5 – Miltenyi has not offered myself, or the CAT Facility, any benefits for this review. This is a work of love.
What the Tyto does
The MACSQuant Tyto is built around the idea of keeping the sample separated from contamination sources – or, inversely, keeping the sample from contaminating anything else. Sorting cells on a MACSQuant Tyto occurs entirely in a closed cartridge that is divided in to three chambers: the input fraction, the sorted fraction and the negative fraction. In the comfort of a sterile tissue culture flow hood, the user will load the sample in to the sample chamber. Once the cartridge is sealed, nothing goes in or out of it until the sort is complete. The user can then bring the cartridge to the instrument and install it in the sample block. To sort the cells, low air pressure (< 3 psi) is applied to the sample, which is then pushed through a fluidic chip. The cells are interrogated by the lasers, the signals are processed, and cells within the population of interest are diverted from the sample stream by a high frequency valve that re-directs the target cell towards the positive chamber. The unsorted flow through continues towards the negative chamber.
It’s a simple design that provides a truly sterile sorting environment and removes any chance of cross-contamination between samples. It also solves the biosafety issue, since there is no chance a sample can reach the operator. However, by sorting in a closed system, you can expect other factors to be modified as well. Light collection and sorting efficiency will be affected. Will that prove to be a deal-breaker?
The Price is Bright
The Tyto has three spatially separated 100mW lasers (488nm, 405nm and 638nm). It can collect signals from up to eight fluorescent detectors: four from the blue line, two from red and two from the violet. There are also three back scatter channels (one off each laser), as well as side scatter off of the blue laser. So, there is still some level of sophistication in the work that can be done. Cell illumination on the Tyto is hampered by the fact that it needs to go through the microchip – meaning there is no hydrodynamic focusing, the cells are instead aligned through a microfluidic line. This is not as sensitive as other common cell sorters, as illustrated by the 8 peak beads test data we collected. While this data comes from the demo instrument—which is likely not in as good shape as it could be—it tells us that the markers used to sort the cells will need to be very bright. There are two reasons for this:
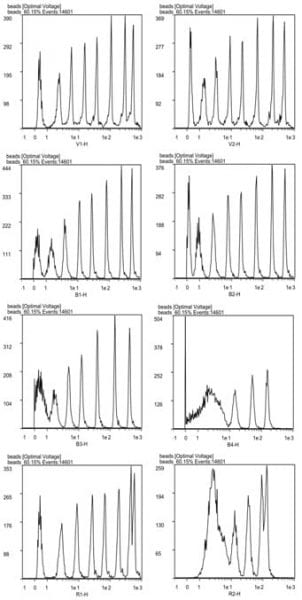
Figure 2. 8 peak bead data run on all detectors of the Tyto. Voltage was selected to provide the best resolution of as many peaks as possible.
1 – The gating strategy will be improved by having the positive and negative fractions well separated. This is a common factor on flow cytometry cell sorters. It is much easier to design an effective gating strategy on a population of cells that is very distinct from other cell clusters. Gating on poorly resolved populations typically results in lower sort purity, i.e. you will be collecting unwanted cells along with your population of interest.
2 – More specifically to the Tyto, the cell staining also plays a role in calculating the time of flight of the cells in the microfluidic chip and ultimately, the efficiency of the sort. The Tyto decides when to activate the high frequency valve by measuring the time it takes for a cell to move from one laser to the next. While the pressure on the sample remains constant (the instrument will modulate the pressure to keep a transit time of 40us between two laser beams, but the operator can’t modify the pressure), some cells might be traveling faster than others based on their size. Preferably, you will want to sort positive events on markers excited by two successive laser beams. Blue and violet excited markers are preferred since they are closest to the sorting valve. Using a dim marker or autofluorescence increases the chance of having the instrument mismatch its signals and sort the wrong cell. Using autofluorescence signals can still work, but may impact sort efficiency.
One side note: as with other chip-based cell sorters, there is no detector on the other side of the chip since light can’t get through.. That means that there is no forward scatter on the Tyto. Instead, the back scatter detector captures the light bouncing back from the chip. This works well enough to identify the main cluster of cells from noise and debris, but is not all that useful to separate cells based only on size. Careful marker selection is needed to sufficiently identify the target population.
Dealing with a high-frequency valve
The high-frequency valve on the Tyto cartridge is capable of 30 000 movements per second. From what we’ve seen during both instrument demo, it can handle the same sample preparation as we get on our Arias and deliver similar results in terms of purity and sorting efficiency. There is a five-year old in me who thinks that the valve might start to mash up the cells if the sort rate gets too high, but Miltenyi’s application specialists mention that at the recommended concentration of 50 million cells/mL or less, they are well separated and should not get hit. Bigger cells run a higher risk of getting clipped and there is a cell size limit on what the cartridge can handle (~20um). Otherwise, unless the flow control is not working properly, nothing should get chopped up.
One consideration is the impact of frequent valve movements on sorting efficiency. While the valve is activated, other cells—which may be our target cells—are flowing through. Thus, the event rate may get high enough that sorting efficiency decreases. The highest recommended sort rate is 5000 cells per second. The flow rate of the sample can’t be modulated by the operator during the experiment, so event rate is mainly controlled by the cell concentration of the sample. In addition, sort purity is also a function of cell concentration on this system. Nicely enough, the sample preparation can be optimized beforehand by calculations based on Poisson distribution. The frequency of the target population of cells and the desired sort purity will dictate the most favorable cell concentration or inversely, the cell concentration and population frequency will predict the sort purity.
What does this means in terms of sorting speed? Can the Tyto keep up with droplet-based sorters? The Tyto will normally run 4mL of sample per hour. By comparison, our rule of thumb for our Aria with a 100um nozzle tip is roughly 2mL of sample per hour at a concentration of 10 million cells per mL. But as we just saw, the sample cell concentration on the Tyto will vary based on the frequency of the fraction that needs to be sorted. So, it gets tricky to compare the two systems. Look at it this way: a head-to-head between an Aria and the Tyto will likely favor the Aria if the sample volume is fairly small. But consider a project from one of our users who needs to sort regulatory T cells out of a leukopak, starting with billions of cells in 50 mL. With the Tyto, we could run a debulk step to quickly enrich the sample and then run the sample a second time to purify the target cells. Compared to the Aria, the result is a 2-3 fold decrease in sorting time needed to run through all that material, while keeping cells at high viability.
Plug and play-ish
The Tyto is said to be plug and play: the user should be able to install the cartridge, load the sorting protocol, and get the instrument running without staff assistance. This is true enough from what we’ve seen. The cartridge is aligned automatically in front of the laser beams and supervision by a specialized operator does not seem necessary to me once the instrument is running. However, setting up a protocol is not as intuitive, and a good understanding of the inner workings of the instrument is needed to optimize the purity and sorting efficiency. Even an experienced flow cytometrist might need a few seconds to figure out the voltage selection, the manual compensation, the threshold, and the Arrival Window settings — a secondary gating set that coordinates the valve activation with the travel time of the cells. But once the protocol is set up, any user can re-open that protocol and run the Tyto on their own for any subsequent experiments. Staff and/or user supervision is not needed during run time because if the sample clogs the fluidic line, it simply stops flowing until the situation is remedied. No spill to clean, no sample loss.
Speaking of clogs, before it goes through the microchip the sample has to go through a 20 μm mesh filter, which will prevent clogs in the fluidics. Like in any sorting experiment, good sample preparation is critical to obtain good data. But on a Tyto, clogs can easily become expensive. The issue is that if the filter or the chip gets clogged, the only solution is to move to a different cartridge. It’s not a bad solution except that cartridges cost money. So, samples should be carefully prepared. Miltenyi offers a sorting buffer that helps reduce cells sticking to each other and therefore reduces clogs. Overall, the same measures taken on the droplet sorters to minimize clumps should be taken here: filtering, DNAse, and Ca2+ free media.
No 561nm laser and everyone agrees it’s a shame
The main target for the Tyto is the clinical setting, which I think explains why you won’t find a 561nm laser needed to excite fluorescent proteins such as mCherry and dTomato. I’m told there is no room to add more lasers at this time and Miltenyi is not offering any variation of optical configuration. I’m guessing that Miltenyi had to go through many hoops to make the Tyto GMP compliant, which probably locks this configuration. Still, I want a 561nm laser in there. Not having the ability to sort all fluorescent proteins on a sorter designed for cell culture is a head scratcher. Given the choice, I’d rather throw out the red laser and replace it with a Yellow-Green line, even if it means getting an APC signal from the 561nm laser. It’s not great, but being able to excite a very wide range of fluorescent proteins that are currently ignored is a very big deal in our research environment. I presented this request to everyone I got to meet in the Miltenyi group and everyone seems to recognize how useful the 561nm laser would be. So hopefully this means it may be a possibility in the near future. That’s usually what happens when I want something. Right?
Would researchers use this sorter?
So is there a path for a successful usage of the Tyto in a core facility? I strongly believe so. There are several groups that would benefit from a sterility-first cell sorter.
1 – Sterile sorts: anyone who needs to culture the sorted cells will have a fool-proof solution to the dreaded contamination issue. While the cost of the cartridge may be a bit of a shock at first, the cost of losing an experiment to contamination makes it quite acceptable in my opinion. I strongly suspect that a portion of our research community just does not trust the Aria when it comes to putting sorted cells back in culture. This instrument is likely to better serve them.
2 – Fragile cells: we have several groups who struggle with cell viability at the back-end of a sort on the Aria. Our go-to solution is usually to lower the sheath pressure, but the ability to go down to 3psi would likely solve most of these issues.
3 – Microbial samples: I’m never excited about running bacterial samples on our sorters for fear of contaminating the sorter itself. Sorting bacteria on the Tyto has its own challenges due to the requirement of bright markers, but a trial experiment that we did this year leads me to believe that it can be done successfully.
4 – Uncommon models: many years ago, we were asked to sort samples coming from insects. The issue was that the PBS running through our sorter would put the cells in osmotic shock, so we had to change the sheath and in-line sheath filter to accommodate that experiment. It was a bit of a pain. On the Tyto, we get to keep the cell in whatever media will keep the cells alive. So we could easily accommodate researchers using these uncommon models.
Overall, the Tyto offers an opportunity to manipulate the cells in a different way than what I’ve come accustomed on the droplet-based sorters. It remains nimble enough that it is not strictly limited to low complexity sterile sorts and provides us with the ability to sort the cells over and over with little impact on viability. Will the price of the cartridge be such a deterrent? I’m willing to bet that with the cost of preparing samples towering over the cartridge price, most researchers will see the benefit in using the right tool for the job.



0 Comments